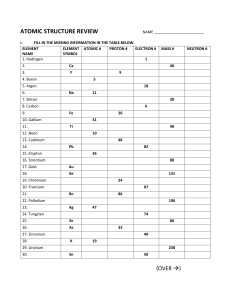
ATOMIC STRUCTURE REVIEW NAME ________________________ I. FILL IN THE MISSING INFORMATION IN THE TABLE BELOW. ELEMENT ELEMENT ATOMIC # PROTON # ELECTRON # NAME SYMBOL 1. Hydrogen 1 2. Ca 3. F 4. Boron 40 9 5 5. Argon 6. 18 Na 11 7. Silicon 28 8. Carbon 9. 6 Fe 10. Gallium 11. 26 31 Ti 12. Neon 48 10 13. Cadmium 14. 48 Pb 15. Krypton 82 36 16. Strontium 88 17. Gold Au 18. Xe 131 19. Chromium 24 20. Francium 21. 87 Rn 86 22. Palladium 23. 106 Ag 47 24. Tungsten 74 25. Br 26. As 80 33 27. Zirconium 28. 40 K 19 29. Uranium 30. MASS # 238 Sn 50 (OVER ) NEUTRON # II. Please answer the following review questions based on what you learned from completing your Chapter 4 Outline. 31. What did Rutherford’s Gold Foil experiment tell us about the modern atom? 32. What part(s) of John Dalton’s atomic theory do not agree with our current model of the atom? 33. Who determined the mass and the charge of an electron? 34. What are TWO ways that isotopes differ from their stable elements? 35. a) How is mass number calculated? b) How does the calculation of “mass number” differ from the way average atomic mass is calculated?