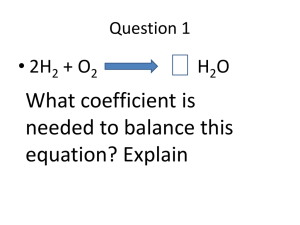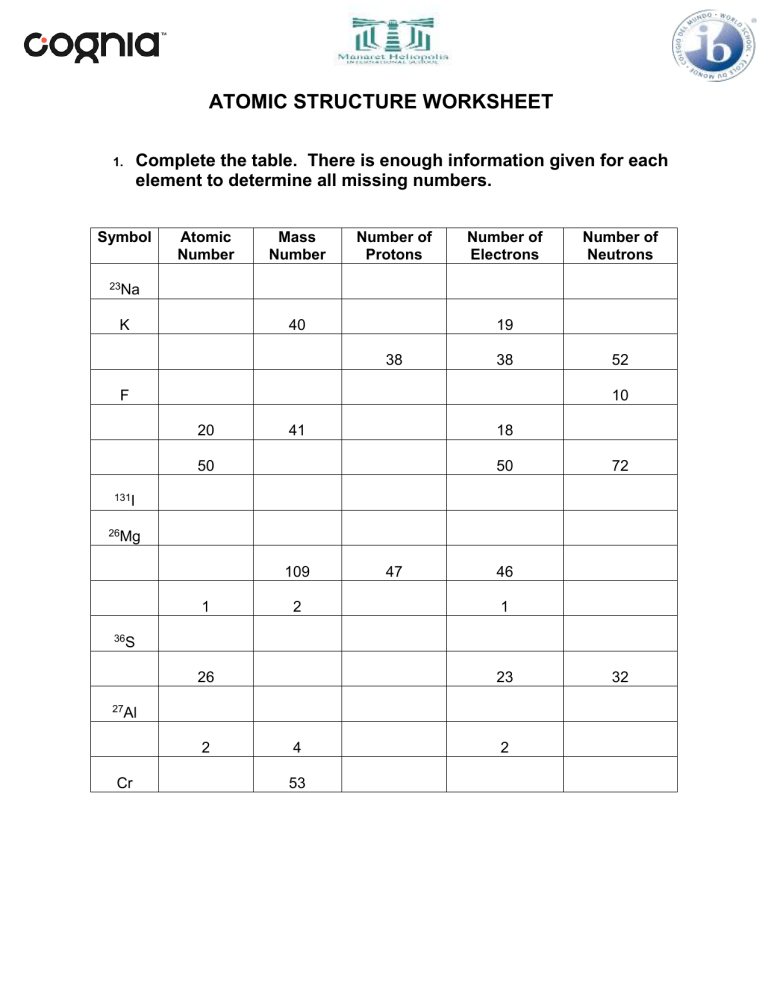
ATOMIC STRUCTURE WORKSHEET 1. Complete the table. There is enough information given for each element to determine all missing numbers. Symbol Atomic Number Mass Number Number of Protons Number of Electrons Number of Neutrons 23Na K 40 19 38 38 F 52 10 20 41 18 50 50 72 131I 26Mg 109 1 2 47 46 1 36S 26 23 27Al 2 Cr 4 53 2 32 2. Use the terms from the following list to complete the sentences below. Each term may be used only once. Some terms may not be used. atom atomic number electron nucleus electron cloud proton isotope neutron 1. A positively charged particle in the nucleus of an atom is called a(n) ______________________ 2. An atom of an element that has the same number of protons, but different numbers of neutrons is called a(n) ______________________. 3. The region in an atom that contains most of the mass is called the ______________________. 4. The number of protons in an atom determines its ______________________. 5. The smallest particle into which an element can be divided and still be the same substance is a(n) ______________________. 3. Complete each of the following sentences by choosing the correct term from the word bank. group period alkali metals halogens noble gases 1. Elements in the same vertical column on the periodic table belong to the same ______________________. 2. Elements in the same horizontal row on the periodic table belong to the same ______________________. 3. Elements that are unreactive are called ______________________. 4. Use the following diagram to answer the following questions: 1. Which letter refers to the negatively charged particles? ……………. 2. Which letter refers to the positively charged particles? ……………. 3. Which letter refers to the particles with no charge? …………… 4. Which letter refers to the center of the atom? ……………. 5. Fill in the blank spaces in the table below which refers to ions of different elements and the fundamental particles present in them. Ion Al3+ K+ 71Ga3+ Atomic no. 13 Neutrons Electrons 14 22 31 37 17 9 10 17 Ti4+ 69Ga3+ 6. Proton s 19 37Cl F35Cl - Mass no. 18 48 18 31 Li + 3 Cu2+ 29 7 34 Describe how an ion of sodium and an ion of chlorine is formed. You can use a diagram. ………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………………….….