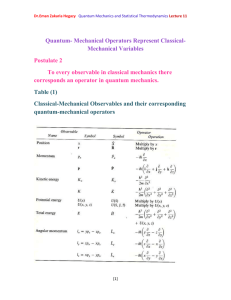
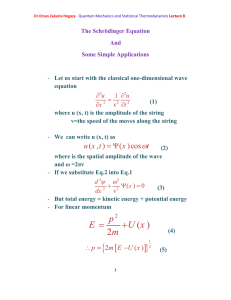
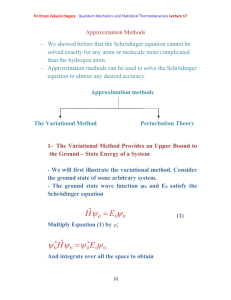
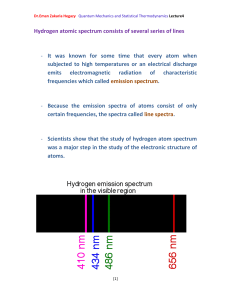
Photoelectric effect Photoelectric effect: a phenomenon in which electrons are
advertisement

Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture3 Photoelectric effect Photoelectric effect: a phenomenon in which electrons are ejected from the surface of some metals exposed to light of at least a certain minimum frequency, called the threshold frequency. - The number of electrons ejected was proportional to the intensity of the light. - Below the threshold frequency no electrons were ejected. - The photoelectric effect could not be explained by the wave theory of light. - If the potential is continuously increased, the electrons will be stopped completely and potential needed to do that called the stopping potential. - At the stopping potential, the initial kinetic energy of the electrons is equal the potential energy. ½ mv2= - e Vs (1- 10) Where Vs: is the stopping potential e: is a negative number 1 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture3 Einstein explained the photoelectric effect with a quantum hypothesis - Einstein appealed to Planck’s hypothesis but extended it in an important way - Planck had applied his energy quantization concept, h. , Planck believed that once the light energy was emitted it behaved like a classical wave. - Einstein proposed instead that the radiation itself existed as small packets of energy , photons. h . , which are known as - Einstein showed that the kinetic enrgy of the ejected electrons ½ mv2 is equal to the energy of the incident radiation h. minus energy required to remove an electron from the surface of particular metal () . 1 m .v 2 h . 2 (1-11) 2 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture3 Where called the work function of the metal , is similar to an ionization energy. Suppose that h . h . o (1-12) Where o : threshold frequency is the minimum frequency that will eject an electron is just a frequency required to overcome the work function off the metal. Using equations (1-10) and (1-12) we can write (1-11) as eV s h . h . o One electron volt = (1 coulomb)× (1 volt) = 1 joul 1eV= (1.602×10-19 C)(1 V) = 1.602×10-19 J 3 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture3 Example 4: Given that the work function for sodium metal is 1.82 eV, what is the threshold frequency? Solution: We must first convert from electron volt to jouls 1.602 1019 ) 2.92 1019 J = 1.82 eV = (1.82eV )( eV . From Eq. (1-12) h .o= h: Planck’s constant: 6.626 × 10-34 J.S 2.92 1019 J 14 1 o 4.40 10 ( S ) 34 h 6.626 10 J / S =4.40×1014 Hz Because Hz= S-1 4 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture3 Example (5): When Lithium is irradiated with light , one finds a stopping o potential of 1.83 V for =3000 A . From these data and known charge on the electron find (a) Plancks’s constant (b) the threshold potential and (c) the work function of lithium Solution: c (a) From eq. (1-13) and e (V 1 V 2 ) h .( 1 2 ) h .c ( 1 1 1 2 ) 1 1 e (1.83 0.80) h 3 10 ( ) 10 10 3000 10 4000 10 8 e (1.03V ) h .(2.5 1014 Hz ) h 1.03V 15 1 4.12 10 J . S . C e 2.5 1014 h (4.12 1015 ) (1.602 1019 ) =6.60×10-34J.S 5 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture3 (b) Using =3000Å eV s h . h . o h. (1.602×10-19C)(1.83 V)= c h . o 10 3000 10 m 6.63 1034 3 108 h . o 7 2.93×10-19= 3 10 h . o 6.63 1019 2.93 1019 h . o 3.7 1019 3.7 1019 14 o 5.57 10 Hz 34 6.63 10 (c) h . o 3.7 1019 J to convert to eV divide on 1.602×10-19 3.7 1019 2.3 eV 19 1.602 10 6