Lecture 4

Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture4
Hydrogen atomic spectrum consists of several series of lines
It was known for some time that every atom when subjected to high temperatures or an electrical discharge emits electromagnetic radiation of characteristic frequencies which called emission spectrum .
Because the emission spectra of atoms consist of only certain frequencies, the spectra called line spectra .
Scientists show that the study of hydrogen atom spectrum was a major step in the study of the electronic structure of atoms.
[1]
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture4
Johann Balmer
Balmer showed that
14
8.22 10 1
4 n
2
Hz n= 3, 4, 5…
He substituted
1
cm -1 called wave number
109.680
1
1
2
2 n
2
Cm -1 (1- 14)
Where n= 3, 4, 5…………………..
This called Balmer formula.
Example (6)
Using Balmer’s formula, calculate the wave lengths of the first few lines of the visible regions of the hydrogen atomic spectrum n=3, n=4
Solution:
The first line is obtained by setting n= 3
109.680
1
1
2
2 3
2
Cm -1
=1.523x10
4 Cm -1
1
λ= 6.564x10
-5 Cm = 6564 Å
[2]
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture4
The next line is obtained by setting n=4
109.680
1
1
2 2 4 2
Cm -1
=2.056x10
4 Cm -1
λ=4.863x10
-5 Cm=4863 Å
The Balmer series occurs in the visible and near ultraviolet regions. There are lines in the hydrogen atomic spectrum in other regions; in fact there are series of lines similar to the
Balmer series in the ultraviolet and in the infrared region.
The Rydberg formula accounts for all the lines in the hydrogen atomic spectrum
Rydberg accounted for all lines in the hydrogen atomic spectrum by generalizing the Balmer formula to
109.680
n
1
1
2
1
2 n
2
Cm
1 ( n
2
n
1
)
(1-15)
This equation is called Rydberg formula.
The constant in Eq 1-15 is called Rydberg Constant
R
H
1
1
2 n n
1 2
2
R
H
is the Rydberg constant = 109.67 Cm -1
[3]
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture4
[4]
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture4
Example 7:
Calculate the wavelength of the second line in the Paschen series and show that this line lies in the near infrared, that is, in the infrared region near the visible.
Solution:
In the paschen series, n
1
=3 and n
2
= 4, 5, 6… According to figure
Thus the second line in the Paschen series is given by setting n
1
=3 and n
2
=5 in Eq. 1-15:
R
H
n
1
1
2
1
2 n
2
Cm -1
109.680
1
1
3 2 5 2
Cm -1
-1
1
Å
[5]
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture4
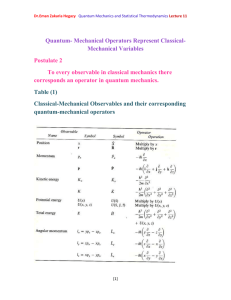
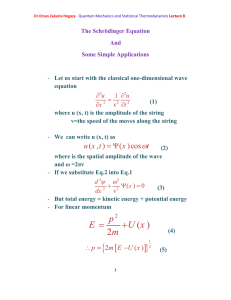
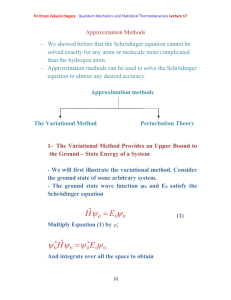
Angular momentum is the fundamental property of rotating systems:
Linear momentum is given by m
and is usually denoted by symbol
. Now consider a particle rotating in a plane a bout fixed centre as in the figure
Let
rot
be the frequency of rotations (Cycles/second)
The velocity of particle v=2πr
rot
= r ω rot
where ω rot
=2π
rot
has units of radians/second and is called the angular velocity. The kinetic energy of the revolving particle is:
[6]
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture4 k
1
2 mv
2
1
2 mr
2
2
1
2
I
2
(1-17)
Where I =mr 2 is the momentum of inertia where
L
I
mr
2 v
( ) r
mvr
(1-18)
Where L is called the angular momentum
Kinetic energy can be written in terms of momentum for a linear system k
mv
2
2
( mv )
2
2 m
P
2
2 m
(1-19)
And for a rotating system k
I
2
2
( I
)
2
2 I
L
2 I
2
(1-20)
F
mv
2 r
(1-21)
Where F : inward force to keep it moving in a circular orbit.
[7]