advertisement
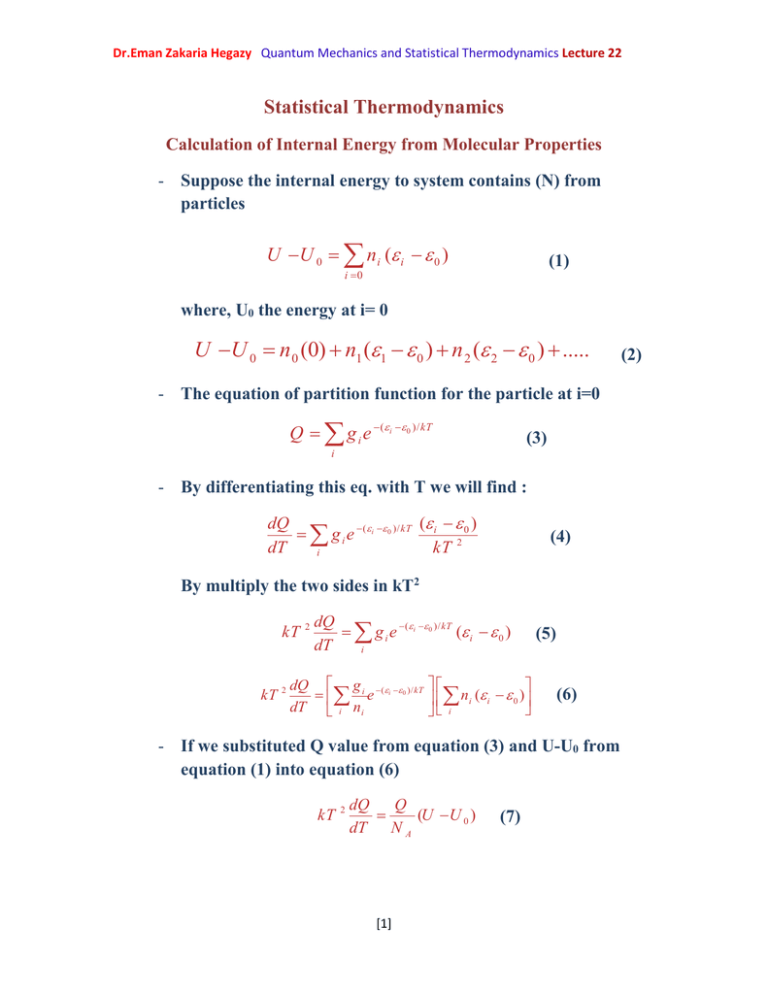
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 22 Statistical Thermodynamics Calculation of Internal Energy from Molecular Properties - Suppose the internal energy to system contains (N) from particles U U 0 n i ( i 0 ) (1) i 0 where, U0 the energy at i= 0 U U 0 n 0 (0) n1 (1 0 ) n 2 ( 2 0 ) ..... - The equation of partition function for the particle at i=0 Q g i e (i 0 )/ kT (3) i - By differentiating this eq. with T we will find : ( ) dQ g i e (i 0 )/ kT i 2 0 dT kT i (4) By multiply the two sides in kT2 kT kT 2 2 dQ g i e (i 0 )/ kT ( i 0 ) dT i dQ g i (i 0 )/ kT e ni ( i 0 ) dT i ni i (5) (6) - If we substituted Q value from equation (3) and U-U0 from equation (1) into equation (6) kT 2 dQ Q (U U 0 ) dT N A [1] (7) (2) Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 22 - where NA is the Avogadro’s number - But k is the Boltzmann constant = R/NA - R=k NA - Substitute R value in equation (7) RT 2 dQ d ln Q (U U 0 ) RT 2 ( ) (8) Q dT dT - Equation (8) is the relation between the internal energy of statistical thermodynamics and the partition function for the particle. - This is clear to us that we can calculate the thermodynamic energy to any type of motion by the value of partition function with temperature. d ln qt q r qv ) dT d ln qv d ln q r d ln qt RT 2 ( )( )( dT dT dT (U U 0 ) RT 2 ( ) (9) (U U 0 ) (U U 0 )t (U U 0 )r (U U 0 )v - The mathematics uses the partition function in three dimensions to calculate (U-U 0)t they found: (U U 0 )trans RT 2 2 mkT 3 d ln ( )V dT h 3 RT 2 [2] (11) (10) Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 22 And also they calculate (U-U0)rot they found as the same: (U U 0 ) rot RT 2 d ln q rot dT (12) 3 RT 2 - Also they calculate the internal energy for the vibrational motion (U-U0)vib (U U 0 )vib (U U 0 )vib d ln qvib RT 2 dqvib RT dT qvib dT 2 (x / T )e x x RT RT (1 e x )2 (e x 1) 2 (13) - This vibration for one molecule but the energy of multi molecules is (U U 0 )vib 3n 6 RT i xi (14) (e x i 1) - The total internal energy is summation of equations (11),(12) and (14) 3n 6 x 3 3 (U U 0 ) RT RT RT x i i (15) 2 2 (e 1) i (U U 0 ) 3RT 3n 6 RT i xi (e 1) (16) xi [3]