Lectuer 14
advertisement

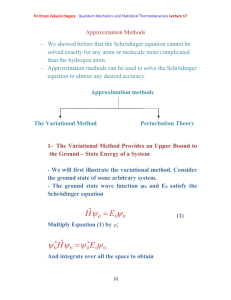
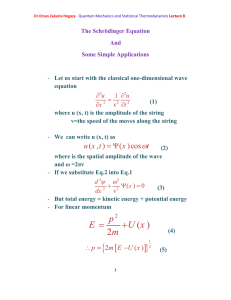
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 14 The Schrödinger Equation for the Hydrogen Atom can be solved exactly - As our model, we shall picture the hydrogen atom as a proton fixed at the origin and an electron of reduced mass µ interacting with the proton through the Coulombic potential U (r ) e2 (1) 4 0 r where e is the proton charge and ε0 is the permittivity of free space , and r is the distance between the electron and the proton. The Schrödinger Equation for the Hydrogen Atom is 2 2 (r , , ) U (r ) (r , , ) E (r , , ) (2) 2 where 𝛁2 written out in full equation 2 is 1 2 r 2 r 2 r r 2 1 sin 2 r sin 1 2 2 2 2 r sin U (r ) (r , , ) E (r , , ) - At first sight, this partial differential equation looks complicated. [1] (3) Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 14 - To solve it first multiply through by 2µr2 to obtain 2 2 r r r 2 1 1 2 sin sin sin 2 2 2 r 2 U (r ) E 0 - The second term in equation can write it as L̂2ψ. Thus we can write the Schrodinger equation in the form 2 r 2 ˆ2 2 r L 2 r U (r ) E 0 (4) r The solution of the Schrodinger equation for the hydrogen atom is a formidable mathematical problem, but is of such fundamental importance that it will be treated in outline here. The solution is managed by separating the variables so that the wave function is represented by the product: (5) Spherical polar coordinates The separation leads to three equations for the three spatial variables, and their solutions give rise to three quantum numbers associated with the hydrogen energy levels. [2] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 14 - When we substitute Lˆ by its value and ψ by R(r) so, equation 4 become d 2 dR r 2 r 2 dr dr 2 2 ( 1) U ( r ) E R (r ) 0 (6) 2 2 r - Equation (6) is called the radial equation for the hydrogen atom. - Equation (6) is an ordinary differential equation in r and must be solved as the wave function , the energy must be quantized according to En - e 4 8 02 h 2 n 2 n= 1, 2,…… (7) 2 2 a h / e If we introduce the Bohr radius, 0 , then 0 Eq.(7) becomes e2 En 8 0a0 n 2 n=1,2,…. (8) - It is surely remarkable that these are the same energies obtained from the Bohr model of the hydrogen atom. - Of course, the electron now is not restricted to sharply defined orbits but is described by its wave function. [3] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 14 - The complete hydrogen wave function are in equation (5) -Table(1) The complete Hydrogen like atomic wave functions for n= 1,2, and 3. The quantity Z is the atomic number of the nucleus, and σ = Z r/a0, where a0 is the Bohr radius. [4]