Quantum Mechanics Operators & Schrodinger Equation
advertisement

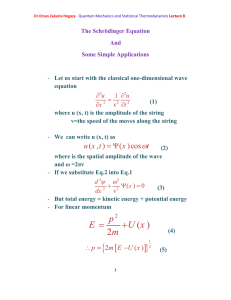
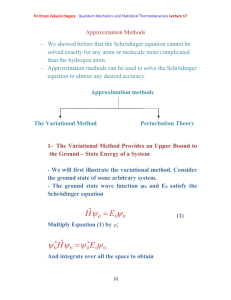
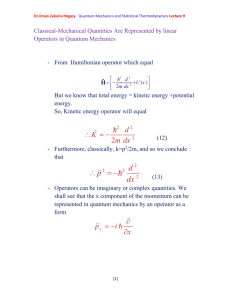
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 11 Quantum- Mechanical Operators Represent ClassicalMechanical Variables Postulate 2 To every observable in classical mechanics there corresponds an operator in quantum mechanics. Table (1) Classical-Mechanical Observables and their corresponding quantum-mechanical operators [1] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 11 Postulate 3 In any measurement of the observable associated with the operator Â, the only values that will ever be observed are the eigenvalues a, which satisfy the eigenvalue equation A a (25) As a specific example, consider the measurement of the energy. The operator corresponding to the energy is the Hamiltonian operator, and its eigenvalue equation is H n E n n (26) This is just the Schrodinger equation. The solution of this equation gives the n and E n . For the case of a particle in a box, En n 2h 2 8ma 2 . Postulate 3 says that if we measure the energy of a particle in a box, we shall find one of these energies and no others. Postulate 4 If a system in a state described by a normalized wave function ψ, then the average value of the observable corresponding to  is given by a A d (27) According to postulate 4, if we were to measure the energy of each member of a collection of similarly prepared system, each [2] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 11 described by ψ, then the average of the observed values is given by equation 27 with Â=Ĥ. The Time Dependence of wave Function is governed by the Time – Dependent Schrodinger Equation Postulate 5 The wave function or state function of a system evolves in time according to the time- dependent Schrodinger equation H (x , t ) i t (28) We can write ψ(x, t) =ψ(x) f (t) Also we take before Schrödinger equation Ĥ ψ (x) =E ψ (x) From these Eqs. We can write f (t) = e –i Et/ħ (x , t ) (x )e iEt / (29) n (x , t ) n (x )e iEn / [3] (30) Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 11 If the system happens to be in one of the eigenstates given by Eq. 30,then n (x , t ) n (x , t )dx n (x ) n (x )dx (31) Thus, the probability density and the averages calculated from eq.30 are independent of time, and the ψ n(x) are called stationary –state wave functions. The Bohr model of the hydrogen atom is a simple illustration of this idea. [4]