Quantum Mechanics: Linear Operators & Free Particles
advertisement

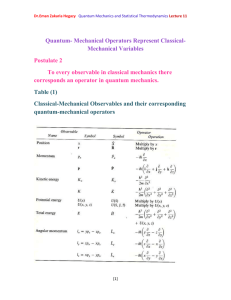
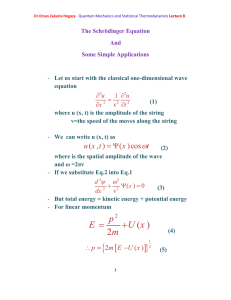
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 9 Classical-Mechanical Quantities Are Represented by linear Operators in Quantum Mechanics - From Hamiltonian operator which equal 2 d2 U (x ) Ĥ 2 2m dx But we know that total energy = kinetic energy +potential energy. So, Kinetic energy operator will equal 2 d2 K 2m dx 2 (12) - Furthermore, classically, k=p2/2m, and so we conclude that p 2 2 d2 dx 2 (13) - Operators can be imaginary or complex quantities. We shall see that the x component of the momentum can be represented in quantum mechanics by an operator as a form p x i [1] x Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 9 The operator p̂2 can be factored as p 2 2 d2 d d (i )(i ) (14) 2 dx dx dx - For example(2): Show that eikx is an eigenfunction of the momentum operator, p x i x . What is the eigenvalue? Solution: We apply p̂x to eikx and find eikx= ħk eikx x and so we see that eikx is an eigenfunction and ħk is an eigenvalue of the momentum operator. p̂x eikx = i Free particle in a one –dimensional box: - In this section we shall study the case of a free particle of mass m constrained to lie along the x axis between x=0 and x=a. This is called the problem of a particle in a one- dimensional box. - The free particle means that the particle experiences no potential energy U(x)=0 - So the Schrodinger equation (7) for a free particle in a one- dimensional box is d 2 2mE 2 (x ) 0 2 dx 0 x a - The particle is restricted to the region cannot be found outside this region. [2] (15) 0 x a and so Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 9 The Energy of a Particle in a Box Is Quantized The general solution of Eq.15 k (2mE ) 1 2 (16) Suppose ka=n π , n=1,2,….. Compare Eqs. 15,16 h 2n 2 E n 8ma 2 n=1,2,…… The energy of the particle is quantized and n is called a quantum number. These wave function are plotted in Figure 1. [3] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 9 Example(3) The model of a particle in a one dimensional box has been applied to the π electrons in linear conjugated hydrocarbons such as butadiene move along a straight line whose length can be estimated as 5.78Å .what is the energy required to make a transition from the n=2 state to the n=3 state Solution: h 2n 2 E n 8ma 2 h2 2 2 E n (3 2 ) 2 8ma (6.6x 1034 J .S )2 5 E n 9.02 1019 J 31 10 2 8(9.1110 kg )(5.87 10 m ) And so we see that this very simple model, called the freeelectron model ,is somewhat successful. [4]