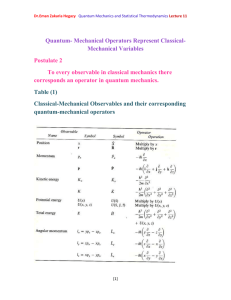
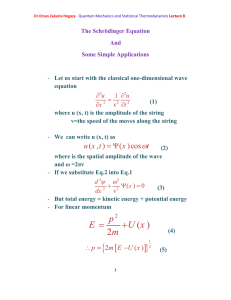
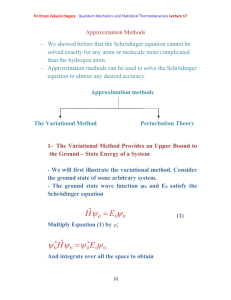
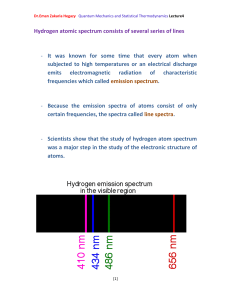
Quantum mechanics
advertisement

Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 1 Quantum mechanics Historical Background of Quantum Mechanics - The development of quantum mechanics began in 1900 in connection with the study of light emitted by heating solids, so we begin by discussing the nature of light. - In 1801Thomas Young gave convincing experimental evidence for the wave nature of light by showing that light exhibited diffraction and interference when passed through two adjacent pinholes - In 1860 Maxwell developed Maxwell’s equations predicted that an accelerated electric charge would radiated energy in the form of electromagnetic waves would radiated energy in the form of electromagnetic waves =c All electromagnetic waves travel at speed c= 3.00×1010 cm/sec in vacuum where : frequency, : wavelength . 1 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 1 II- Black body radiation: The radiation given off by material bodies when they heated, when the burner of an electric stove is heated , it first turns a dull red then white and blue. In terms of frequency, the radiation goes from a lower frequency to a higher frequency as the temperature increases ,because red in a lower frequency region of the spectrum than is blue. Black body = an ideal body which absorbs and emits all frequencies and the radiation emitted by a black body is called blackbody radiation. 2 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 1 *A plot of the intensity of black body radiation versus wavelengths for several temperatures is given in figure (1) *Many theoretical physicists tried to derive expression but they were all unsuccessful, the expression that is derived according to the laws of nineteenth century is by Rayleigh and Jeans Law dv(T)d (8πBT)c3. deq(1-1) where v(T)d is the radiant energy density between frequencies and +d and has a unit s J/m3 ,T is the absolute temperature , and c is the speed of light. The quantity kB is called Boltzmann constant, KB = R/n J/K R: Molar gas constant n: Avogadro’s constant = 6.022× 1023 mol-1 3 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 1 according to this hypothesis the energy of a wave is related to its amplitude and the amplitude varies continuously from zero on up; hence physicists expected the energy of an atom to vary continuously , so they show correlated to physicists cannot this equation was explain the blackbody radiation. III-Black body radiation and energy quantization: Planck assumed that the radiation emitted by the body was due to the oscillations of the electrons in the constituent particles of the material body. Planck able to derive the equation: d(T)d 8πh c3 d(eh/KT-1) eq (1-2) h:planck’s constant = 6.63×10-34 J.Sec This equation is called planck’s distribution Law for Black body radiation. 4