Examples on blackbody radiation
advertisement
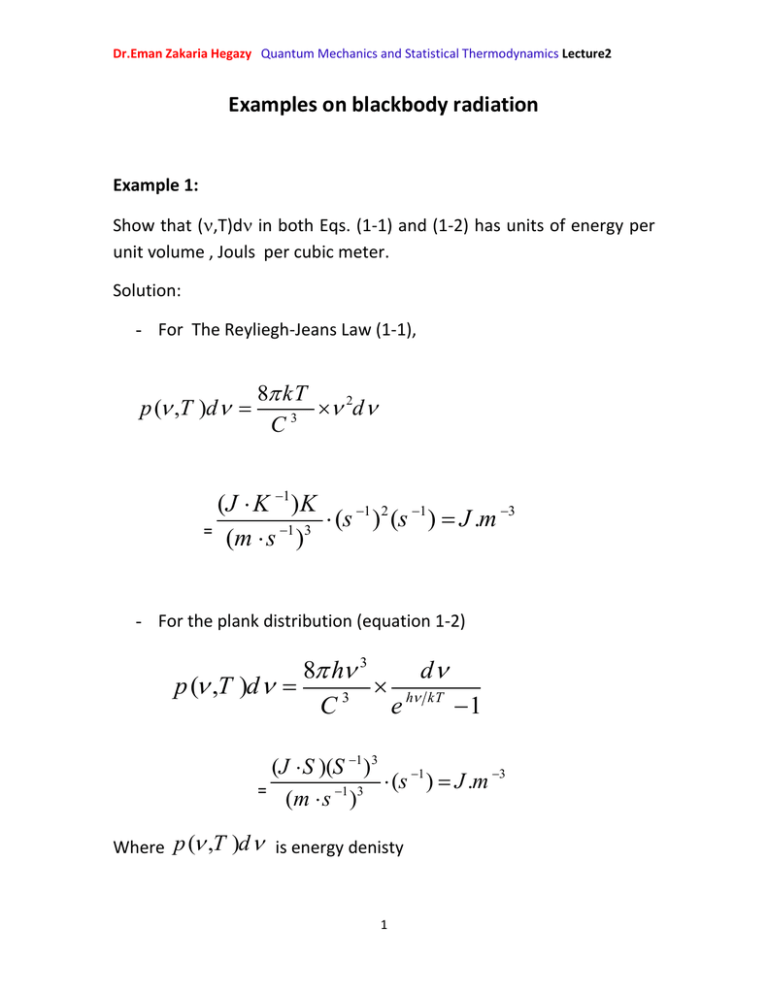
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture2 Examples on blackbody radiation Example 1: Show that (,T)d in both Eqs. (1-1) and (1-2) has units of energy per unit volume , Jouls per cubic meter. Solution: - For The Reyliegh-Jeans Law (1-1), p ( ,T )d 8 kT 2 d 3 C (J K 1 )K 1 2 1 3 ( s ) ( s ) J . m = (m s 1 )3 - For the plank distribution (equation 1-2) 8 h 3 d p ( ,T )d C3 e h kT 1 (J S )(S 1 )3 1 (s ) J .m 3 1 3 = (m s ) Where p ( ,T )d is energy denisty 1 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture2 Example (2): Equation (1-2) expresses planck’s radiation Law in terms of frequency. Express Planck’s constant radiation law in terms of wave length. Solution: h=c, d c d 2 Substitute d into equation (1-2) ( ,T )d 8 hc 5 d e hc kT 1 (1-3) Where ( ,T )d is the energy density between and +d * we can use eqn. (1-3) to derive an empirical relationship was known at time as Wien displacement law. * The Wien displacement law: Says that if max is the wavelength at ( ,T ) is a maximum , then: maxT=2.90 10-3 m.K (1-4) 2 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture2 By differentiating ( ,T ) with respect to hc maxT= 4.965K (1-5) Ex. If we have max = 500 nm T 2.90 103 max 2.90 103 5800K 9 500 10 * Steven-Boltzmann Law: From eq. (1-2) Stefan-Boltzmann showed hat the total energy radiated per unit area per unit time from a black body is given by R C E T 4 4 (1-6) Where E is the total radiation energy is the Stefan-Boltzmann Constant The experimental value of is 5.6697 10-8 J.m-2K-4. S-1 3 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture2 Example 3 Planck’s distribution of black body radiation gives the energy density between n and +d. Integrate the Planck distribution overall frequencies and compare the result to Eq. (1-6) Solution: The integral of q. 1-2 over all frequencies is 8 h 3d E ( ,T )d 3 h kT c 0e 1 0 (1-7) If we use the fact that : x 3dx 4 0 e x 1 15 8 5 k 4T 4 E 15h 3c 3 (1-8) c E T 4 From eq. (1-6) 4 by substituting E from (1-8) into (1-6) 8 5 k 4T 4 c T 3 3 15h c 4 4 4 Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture2 2 5 k 4 15h 3c 2 (1-9) Using the values of k, h and c , the calculated value of is 5.670 10-8 J.m-2K-4.S-1, in excellent agreement with the experimental value. 5