Naming
advertisement

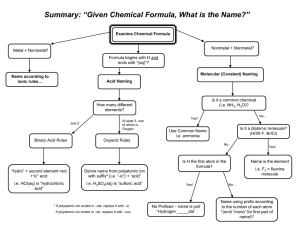
Naming with Polyatomic Ions Naming with Polyatomic Ions On the bottom of Page 1 of your Reference Table is a listing of “Selected Polyatomic Ions” Please refer to it. Naming with Polyatomic Ions Polyatomic Ions: - ions made of two or more atoms - this charged group is considered to be a single ion - form compounds in the same manner as the simple ionic compounds we have studied Naming with Polyatomic Ions Example: Ammonium ion Formula: The +1 charge is spread over the whole polyatomic compound, so it is considered a +1 ion Naming with Polyatomic Ions Polyatomic ions can have different endings than “ide” depending on how many oxygen atoms they contain. Look at nitrite and nitrate ions. What is the difference between formulas? Naming with Polyatomic Ions Look at Sulfite and Sulfate. What is the difference in their formulas? What rule can we make about naming with different numbers of oxygen atoms? Naming with Polyatomic Ions Practice naming the following: Na2SO3 ________________ CaSO4__________________ Write the formula for: Ammonium Nitrate: Sodium hypochlorite: Naming with Polyatomic Ions Finally, when hydrogen is present in a polyatomic ion, we name it. Example: HCO3-1 – NOT HOLLISTER CO. Correct name: hydrogen carbonate HSO4-1 - hydrogen sulfate Naming Compounds Containing Transition Metals Roman Numerals • Keep in mind: V = 5 and X = 10 ☺Rule: If there is an I before V, it means SUBTRACT 1 from 5: 5 – 1 = 4 so IV = 4 ☺Rule: If there is an I after V, it means ADD 1 to 5: 5+1=6 so VI = 6 The same goes for X: IX = 9 Roman Numerals • Roman numerals are used to name ionic compounds in which the metal has more than one oxidation state. • How to count in Roman Numerals: I =1 V =5 IX = 9 II = 2 VI = 6 X = 10 III = 3 VII = 7 IV = 4 VIII = 8 The following are common transition metal ions and their oxidation states: Chromium +2,+3 Copper +1,+2 Iron +2,+3 Lead +2,+4 Nickel +2,+3 Tin +2,+4 Mercury +1,+2 Naming Transition Elements Compounds - Since these metals have a number of different oxidation states, they have to be named differently. - The oxidation state MUST appear in the name so we know which oxidation state we are dealing with. Naming Transition Elements Compounds • The Roman numerals we will deal with most are: I = 1 II = 2 III = 3 IV = 4 Example: Iron Fe2+ and Fe+3 FeSO4 is from: Fe+2 and SO4-2 (sulfate ion) The name of this compound : Iron (II) Sulfate Fe2(SO4)3 is from: Fe+3 and SO4-2 The name of this compound: Iron (III) Sulfate What’s difference does it make? • As an example, the melting point of Iron (II) Sulfate is 300 deg C • The melting point of Iron (III) Sulfate is 400 deg C • You hemoglobin requires the Fe+2 ion to carry oxygen through you body; Fe+3 won’t work! Iron (II) ion in Heme Due to the structure of this molecule, O2 can only “fit” with Fe+2 ions. “Oddly” Named Elements Copper - Symbol Cu - from the Latin word Cuprum - Discovery dates from prehistoric times Elements with “odd” symbols • Gold – Chemical symbol: Au – From the Latin word “aurum” Mercury - Chemical symbol: Hg - from “hydragyrum” - meaning “liquid silver” Elements with “odd” symbols • Silver – Chemical symbol: Ag – From the Latin word “argentum” Lead - from the Latin “plumbum” Elements with “odd” symbols • Iron – Chemical Symbol: Fe – from the Latin “Ferrum” Tin - Chemical Symbol: Sn - from the Latin “Stannum” Elements with “odd” symbols • Sodium – Chemical Symbol: Na – From the Latin “natrium” • Tungsten • Chemical Symbol: W • From the word “Wolfram” which is the ore it is found in