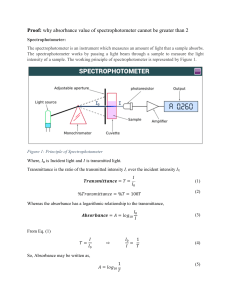
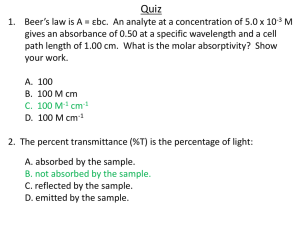
Spectrophotometry Worksheet: Color, Absorbance, Dilutions
advertisement

Spectrophotometry Worksheet Name_______________________ 1. Below are listed either ranges of light or colors of light. In the instance of a range, state what color of the visible spectrum that you would expect to see. In the instance of a color, please state what range of wavelengths transmits that particular color. Green Red 380‐430 nm 590‐620 nm 2. Please define the terms “transmittance” and “absorbance.” 3. When you needed to set the spectrophotometer to 0% transmittance initially, what, if any, sample did you have inside of it? When you needed to set the spectrophotometer to 100% transmittance, what if any, sample did you have inside of it? 4. Please define the term “serial dilution.” Why did we use serial dilutions in the spectrophotometry lab? 1 5. You start with a solution that is 60 mg/ml of protein and perform a series of serial dilutions on it. Please fill in the table below with the appropriate concentrations at each dilution. Concentration (mg/ml) Absorbance 1 60 .750 2 .340 3 .180 4 .08 5 .02 6. You have a mystery solution that has an absorbance of .250. Using just the table in question 5 alone, give an approximation or a range of concentrations that could be that or those of the mystery solution. Please justify your answer. 7. Now graph your results from question 5 on the graph provided on the next page, draw a best fit line, and then determine an approximate value for the concentration of the mystery sample. 2 3