Chem 211 Laboratory
advertisement

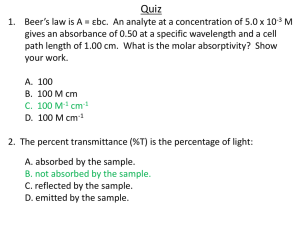
Absorption Spectroscopy CHEM 251 Week of November 1st, 2010 Alexis Patanarut CHEM 251 Laboratory For the week of November 8th, 2010: • Experiment: VSEPR experiment, pp. 99-105 • Due next week: Absorption Spectroscopy lab report • Due this week: Ideal Gas Law lab report Overview • Today, a spectrometer will be used to determine the wavelengths of visible light absorbed by substances in solution. • After the wavelength of maximum absorption is found, a series of standard solutions will be prepared. – From the data obtained, a calibration curve will be plotted to relate the intensity of absorbance and the concentration of a solution. – From this curve, the concentration of an unknown can be determined. Class Data 1.000 y = 4.8793x - 0.0342 R2 = 0.9924 0.800 absorbance 0.600 y = 1.9526x + 0.0085 R2 = 0.9931 group 1 group 2 0.400 Linear (group 1) Linear (group 2) 0.200 0.000 0.000 0.050 0.100 0.150 -0.200 concentration (M) 0.200 0.250 Absorbance = -log (% transmittance/100) Procedure Part I – Calibrating the instrument 1. Turn on the spectrometer and set to 0% transmittance using the left knob. 2. Place a cuvette ½ filled with H2O in the sample holder and set to 100% transmittance with the right knob. 3. Remove the blank. The display should now read 0% transmittance. If not, repeat steps 1-3. Part II – Absorbance vs. Concentration 1. Prepare two 10mL standard solutions from the sample stock solution. Solution 1: ~3mL stock solution Solution 2: ~7mL stock solution 2. Set the instrument to your lmax and calibrate the instrument. 3. Read the absorbance of each of the four solutions (two standard solutions, distilled water, and stock solution) at lmax. Solution lmax: Cu2+ – 625nm; Co2+ – 515nm; Ni2+ – 395nm Absorbance = -log (% transmittance/100) Procedure Part III – The Unknown 1. Obtain a sample of unknown concentration and measure its absorbance at lmax. Be sure to record the number of your unknown. • Do NOT empty the unknown test tubes. 2. Once all of the wavelengths, volumes and absorbances have been recorded, enter the appropriate values in the webbased data entry form. Come see me to get the concentration for your unknown. 3. Return all cuvettes and unknown test tubes to me when you’re done. For the lab report • You should have a total of two data tables and one graph: 1. 2. Your data table (no class data this week!) 1 X-Y Scatter plot showing the Calibration Curve • Show sample calculations for the molarity of your standard solutions and the percent error. • Answer all of the discussion questions from the handout in the Discussion/Conclusion section of your lab report. – Ignore the question about the spectrum graph, since we’ve already found the lmax for you