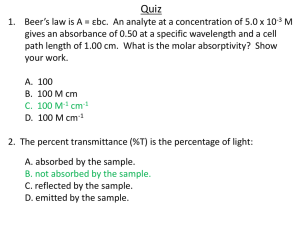
Proof: why absorbance value of spectrophotometer cannot be greater than 2 Spectrophotometer: The spectrophotometer is an instrument which measures an amount of light that a sample absorbs. The spectrophotometer works by passing a light beam through a sample to measure the light intensity of a sample. The working principle of spectrophotometer is represented by Figure 1. Figure 1: Principle of Spectrophotometer Where, 𝐼0 is Incident light and 𝐼 is transmitted light. Transmittance is the ratio of the transmitted intensity I, over the incident intensity I0, 𝑻𝒓𝒂𝒏𝒔𝒎𝒊𝒕𝒕𝒂𝒏𝒄𝒆 = 𝑇 = 𝐼 𝐼0 %𝑇𝑟𝑎𝑛𝑠𝑚𝑖𝑡𝑡𝑎𝑛𝑐𝑒 = %𝑇 = 100𝑇 (1) (2) Whereas the absorbance has a logarithmic relationship to the transmittance, 𝑨𝒃𝒔𝒐𝒓𝒃𝒂𝒏𝒄𝒆 = 𝐴 = 𝑙𝑜𝑔10 𝐼0 𝐼 (3) From Eq. (1) 𝑇= 𝐼 𝐼0 𝐼0 1 = 𝐼 𝑇 ⇨ (4) So, Absorbance may be written as, 𝐴 = 𝑙𝑜𝑔10 1 𝑇 (5) 𝐴 = 𝑙𝑜𝑔10 100 %𝑇 (6) 𝑎 Using division rule of Log 𝑙𝑜𝑔10 𝑏 = 𝑙𝑜𝑔10 𝑎 − 𝑙𝑜𝑔10 𝑏 𝐴 = 𝑙𝑜𝑔10 100 = 𝑙𝑜𝑔10 100 − 𝑙𝑜𝑔10 %𝑇 %𝑇 (7) So, 𝐴 = 𝑙𝑜𝑔10 100 − 𝑙𝑜𝑔10 %𝑇 (8) 𝐴 = 𝑙𝑜𝑔10 102 − 𝑙𝑜𝑔10 %𝑇 (9) 𝐴 = 2𝑙𝑜𝑔10 10 − 𝑙𝑜𝑔10 %𝑇 𝐴 = 2 − 𝑙𝑜𝑔10 %𝑇 ∴ 𝑙𝑜𝑔10 𝑎2 = 2𝑙𝑜𝑔10 𝑎 (10) ∴ 𝑙𝑜𝑔10 10 = 1 (11) 𝐴 = 2 − 𝑙𝑜𝑔10 %𝑇 (12) The last equation, A = 2 - log10 %T , is worth remembering because it allows you to easily calculate absorbance from percentage transmittance data. The value of transmitted light %𝑇 varies from 0 to 100. Case 1: When no light pass through sample i.e. %𝑇 = 0, 𝐴 = 2 − 𝑙𝑜𝑔10 0 = 2 − 0 = 2 (13) Case 2: When all incident light passes through sample i.e. %𝑇 = 100, 𝐴 = 2 − 𝑙𝑜𝑔10 100 = 2 − 𝑙𝑜𝑔10 102 = 2 − 2𝑙𝑜𝑔10 10 = 2 − 2 = 0 (14) It is clear from both cases that the maximum value of Absorption, 𝐴 = 2. Hence proved that the absorption value of spectrometer cannot be greater than 2. 𝑨𝒃𝒔𝒐𝒓𝒑𝒕𝒊𝒐𝒏, 𝑨 ≤ 𝟐 The relationship between absorbance and transmittance is illustrated in the following diagram which shows that the value of Absorbance is less than or equal to 2 for all values of transmittance: