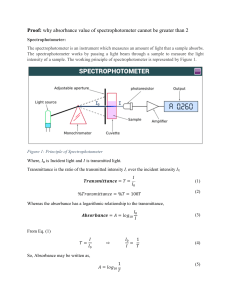
Spectrophotometry Lab: Blue Dye in Gatorade
advertisement

Lab 4 Write up – Spectrophotometry and Dilutions: How much blue dye #1 is in Gatorade? Purpose/Question ___/5 Data and Observations Include Excel Data Table Include Excel Graph with trendline, equation, and R2 value showing Include your qualitative observations of 3 molarities of solutions. _____/10 Calculation Framework Calibration Curve Molarity Calculations (Don’t redo this. You’ve already done it) Determine the molar concentration of blue dye #1 in both Gatorades. Use your line of best fit to accomplish this. Determine the mass of blue dye #1 found in a 32 oz (591 mL) container of both Gatorade Frost and G2. The molar mass of blue dye #1 is 793 g/mol _____/17 Claims ____/5 Evidence ___/5 Conclusion Purpose Recap and description of the two new lab techniques (how to perform a dilution and how to use a spectrophotometer) Nanoscopic description (WITH PICTURES) of different concentrations of solutions AND how a spectrophotometer works. Answer the following questions: o Suppose a solution was too concentrated for an accurate reading with the spectrophotometer. The concentrated solution was diluted by placing 1.00 mL of the concentrated solution in 4.00 mL of water. The solution was then placed in the spectrophotometer and an absorbance was obtained and after a few calculations the molar concentration was calculated to be 3.5 x 10-6 M. What was the concentration of the original stock solution before dilution? o If a 0.10 M solution of a colored substance has a maximum absorbance at 500 nm and an absorbance of 0.26 M at this wavelength, what will be the measured absorbance of a 0.20 M solution at 500 nm? o Mr. Reimer’s good friend Mr. Madonna was conducting the same experiment that we conducted, but forgot to wear gloves while he was handling cuvettes. He inadvertently left fingerprints all over the cuvettes when he ran his data. How would this affect Mr. Madonna’s absorbance readings? His % transmittance? Explain. _____/20 ___/62