Functional Groups
advertisement

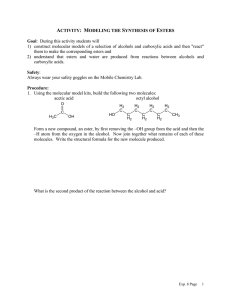
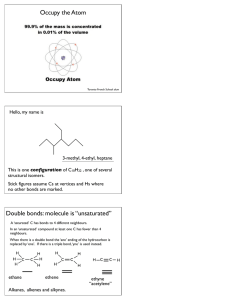
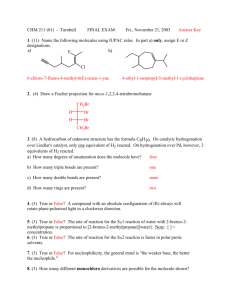
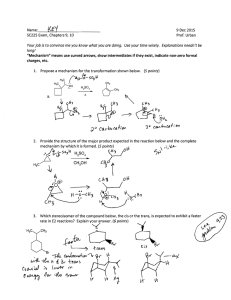
CH 320M/328M Functional Groups A functional group is a defined grouping of atoms in an organic molecule. A given functional group exhibits a characteristic set of chemical properties, which are largely independent of the rest of the structure of the molecule in which it is found. As a consequence, an understanding of the chemical behavior of the functional groups allows one to predict the reactivity of a wide variety of organic molecules. We will encounter numerous functional groups throughout our study of organic chemistry. A few of the more important ones are listed in the table below (continued on the next page). At this stage, it is most important for you to learn to recognize the general structures associated with these functional groups. In each case, a simple example is provided as an illustration. (Note: A line indicates a single bond to carbon or hydrogen.) Functional Group – General Structure Example C CH3CH3 C OH Alkane Ethane Alcohol Ethanol C C CH2=CH2 C O C CH3OCH2CH3 Alkene Ethylene Ether Ethyl methyl ether C C H C C H Alkyne Acetylene Aromatic Ring (Arene) Functional Group – General Structure C SH Example CH3CH2OH CH3CH2SH Thiol Ethanethiol C S C CH3SCH3 Benzene Sulfide Dimethyl sulfide CH3CH2Br C N CH3CH2NH2 Ethyl bromide Amine Ethylamine C X (X = F, Cl, Br, I) Alkyl Halide CH 320M/328M Functional Group – General Structure Example O C O C O C H Aldehyde H3C O C C Ketone H3C O C Carboxylic acid Acid chloride OCH2CH3 Ethyl acetate O C Acetic acid Amide O C CH3 O C O C N H3C O H 3C Ester O C Acetic anhydride C O OH H3C O C Cl Acid anhydride CH3 O C OH O C H 3C O O C Acetone Example O C H Acetaldehyde O C Functional Group – General Structure C C N H 3C NH2 Acetamide H3C C N Cl Acetyl chloride Nitrile Acetonitrile Since the ability to recognize these functional groups, particularly when they are incorporated into a larger, more complicated molecule, is so important, you should learn the names and general structures of all of the functional groups on this handout as soon as possible.