Document 10409930
advertisement

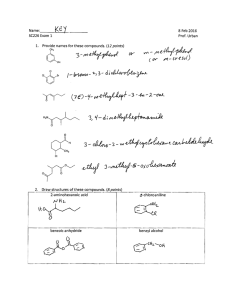
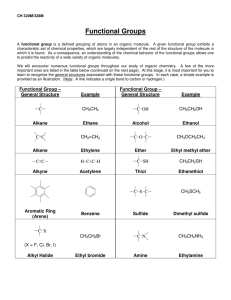
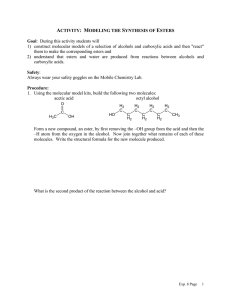
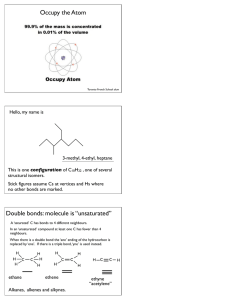
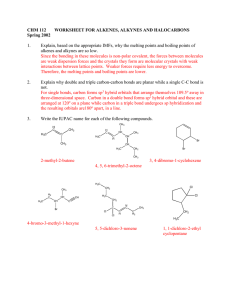
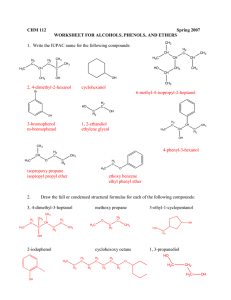
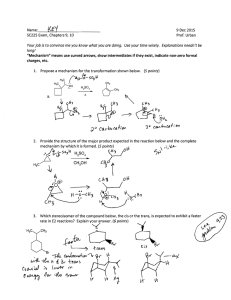
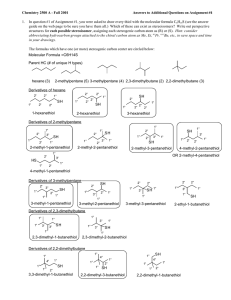
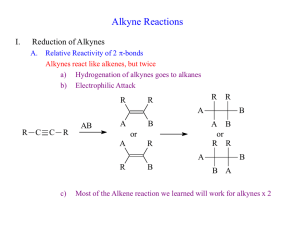
Chem 343 – Organic Reactions Chapter 14 Prepared by José Laboy, MS http: www.chem.wisc.edu/areas /clc (Resource page) Synthesis and Reactions of Alkynes #5: Addition of HX to Alkynes Mechanism H 3C C X H3C C CH HX (1 equiv.) H3C X δ δ X H3C C CH H X δ δ X CH C CH2 X X H3C CH2 X X = Cl or Br When HX is in excess C CH2 HX (excess) H 3C H C H X H3C X X C C CH3 H3C CH3 X X most contributing The reaction of the alkyne in the presence of HX occurs with a overall third order kinetic rate law, where the HX is second order. The product of the reaction follows a Markovnikov addition. It is likely that the more substituted alkyne carbon exhibits a somewhat positive charge in the transitions state. The addition of HX in the second reaction is rather slow compared to the first addition and also follows Markovnikov addition. Consider that the 2π bonds are orthogonal to each other, that is, they are 90° apart. This means that their chemical properties are “somewhat” independent, especially for the first reaction.