Document 11123971
advertisement
l.(€'(
Name:
SC225 Exam, Chapters 9, 10
9 Dec 2015
Prof. Urban
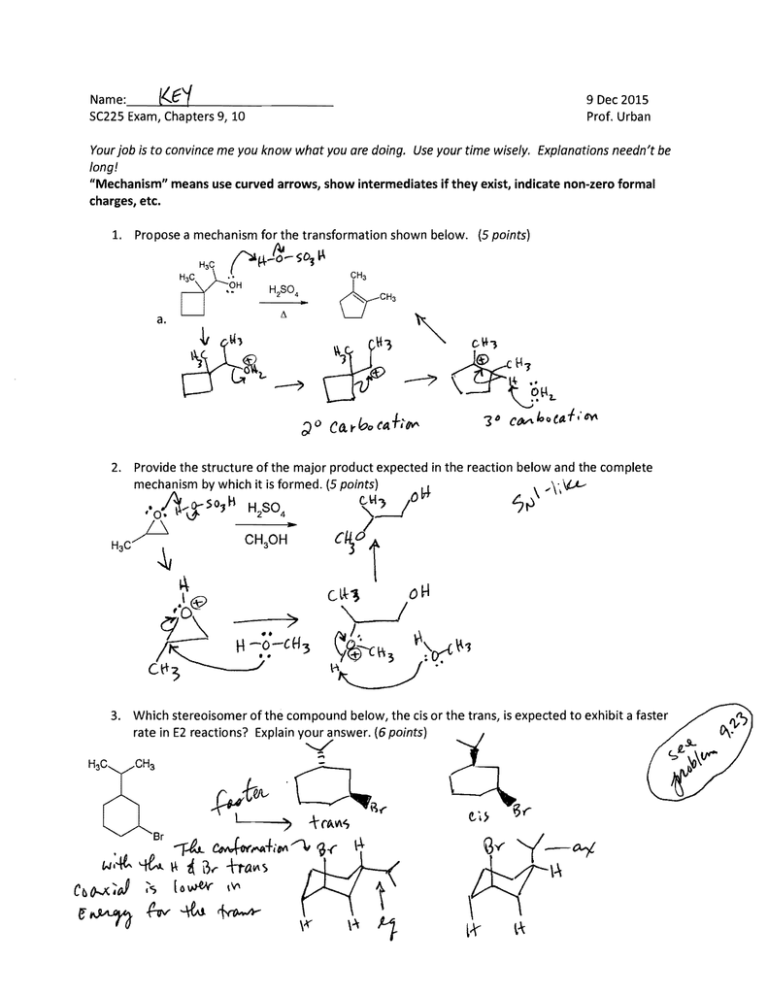
Your job is to convince me you know what you are doing. Use your time wisely. Explanations needn't be
long!
"Mechanism" means use curved arrows, show intermediates if they exist, indicate non-zero formal
charges, etc.
(5 points)
2. Provide the structure of the major product expected in the reaction below and the complete
mechanism by which it is formed. (5 points)
~
\ ,\"\~
)1 ~
. J). . . o-so,t1
H3 c
~,
H SO
C~30~·
/J
c~d'r
L]~
~
.' @
qO\ -
u
>
~~-CH3
Ct+'3
3. Which stereoisomer of the compound below, the cis or the trans, is expected to exhibit a faster
rate in E2 reactions? Explain your answer. (6 points)
V...
\~
4. Provide structures of the alkyl halides that would serve as the best starting materials for the
synthesis of these alkenes in one step with minimal unwanted side products.
(6 points)
a.
5. When the alcohol below is treated with HBr, compound A is the major product. When the
alcohol is treated with H2S04, a different compound, B, is produced as the major product.
y;; HBr
B
A
a.
b.
Provide structures of A and B.
Explain why use of hydrobromic acid and sulfuric acid leads to different products. (i.e.,
what difference in the acids is responsible for the difference in the products?)
(8 points)
fh .so of
'6.A.k
-c., +e. !3~ ~
1+ ~v ~ ~f /1 -tr
vwc,&.
rf:h
(f!x- -) J-)
tJ.--­
$/A
f.s 1-; !wI;;;,."
6.
Fill in the blanks. Provide the missing reactant(s), product(s), or reagent(s). (3 points each)
q
O)l..
Bf
~
~
DMSO
NaOH
..
?
NaOH
?
~OTs
..
?
?
B'~
H+Br
Br+H
..
CHpH
?
+ 2 NaNH2
-
?
Ph
o
H3C
'N(CH3h
NaOH·
?
SOCI 2
..
~t1
NaCN
..
DMSO
?
cro3cr"O
?
'"'
~o
..
CH 2CI 2
?
~(P,~
\-",.1 C!{1
?
..
NaOEt
EtOH.8
?
'2..
OC. l-tl
at" - d"
7.
For each transformation shown below, state if it is an oxidation or a reduction. (4 points)
0)(;
d•.+. CJV'
(
i t'I C ~ t___
.',
0
i.M"..J
8. Provide structures of the alkyl halide and alkoxide ion needed to produce the ether below via
Williamson Ether Synthesis. (6 points)
UO'CH,
09.
0
Which substrate below is expected to give the highest ratio of substitution-to-elimination
products in reaction with sodium ethoxide in ethanol? (4 pOints)
)-Br
10. Which reaction conditions will result in the highest ratio of elimination-to-substitution products
with bromocylcohexane as the substrate? (4 points)
o
-O.Jl CH3
.. EtOH
..
EtOH
NaOEt
NaBr
EtOH
EtOH
.$ 1YlI't'j
..h~
1
~1/\Ckrf.J 11. Will it work? Each of the proposed syntheses below mayor may not contain a design flaw. In
each case, explain if the proposed syntheses is expected to work as written. If there is a flaw in
the synthetic design strategy, explain what the problem is. (8 points)
H3C~Br
HC::;:::CNa
.
•
.::6CH
H3C~:;7
1. NaNH 2, NH 3(1)
2.
L>-CH
..
3
H3C~
OH/
3.W
~CNa
t
12. Provide the reactions needed to perform the following synthetic transformations. (8 points)
~NH2