Chem: PS D14
advertisement

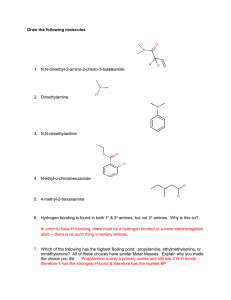
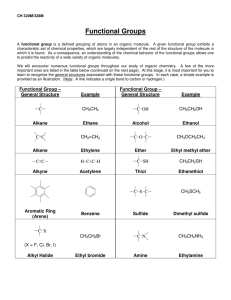
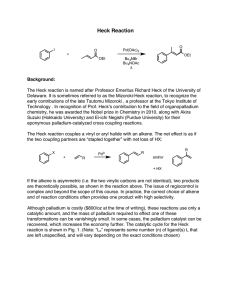
Chemistry Problem Set: D14 Dalton’s Law and Graham’s Law Block: ____ Name: ________________ The next Quest homework assignment (HWK 3) is due on Tuesday Feb. 19th at 8 am. Remember, no school on Monday Feb. 18th! Read 14.4 pages 432-436. 1. Suppose you had a mixture of oxygen, nitrogen, and helium. The partial pressures of the gases were: PO2 = 731 torr; PN2 = 46.1 kPa; and PHe = 0.45 atm. What’s the total pressure of the mixture in mm Hg? 2. A typical sample of Martian “air” is primarily composed of 95.86% CO2, 2% Ar, 2% N2; and 0.14% O2. If the total atmospheric pressure is 0.60 kPa, what are the partial pressures for each of these molecules? 3. KMT says that the average kinetic energy =(3/2)nRT where R is the universal gas constant and T is the temperature in Kelvin. But, we also know that the average kinetic energy = ½ mv2 where m is the mass of a molecule and v is the average velocity. Consider the molecules O2, H2 and CH4 in separate 1 L containers at 298 K. a. Which molecule has the largest average kinetic energy? b. Which molecule is moving the fastest? Which the slowest? 4. Samples of ethyl butanoate (chemical formula CH3CH2CH2COOCH2CH3) and ethyl acetate (chemical formula CH3COOCH2CH3) are left open to evaporate. Ethyl butanoate smells like pineapple and ethyl acetate is the smell in nail polish remover. a. What are the molecular weights of these two compounds? b. Which compound will diffuse through the room more quickly? Explain. 5. A balloon has equal amounts of hydrogen gas and an unknown gas in it. Suppose that the hydrogen effuses through the pores of the balloon exactly 10 times faster than that of the unknown gas. What is the molecular weight (molar mass) of the unknown gas? Answers (IRO+1): 200 0.5752 88.105 0.0120 116.16 112.31 0.012 0.00084 1418.9