A : M S
advertisement

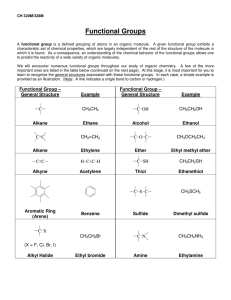
ACTIVITY: MODELING THE SYNTHESIS OF ESTERS Goal: During this activity students will 1) construct molecular models of a selection of alcohols and carboxylic acids and then "react" them to make the corresponding esters and 2) understand that esters and water are produced from reactions between alcohols and carboxylic acids. Safety: Always wear your safety goggles on the Mobile Chemistry Lab. Procedure: 1. Using the molecular model kits, build the following two molecules: acetic acid octyl alcohol O H2 C C H3C OH HO H2 C C H2 H2 C C H2 H2 C C H2 CH3 Form a new compound, an ester, by first removing the –OH group from the acid and then the –H atom from the oxygen in the alcohol. Now join together what remains of each of these molecules. Write the structural formula for the new molecule produced. What is the second product of the reaction between the alcohol and acid? Exp. 8 Page 1 2. Follow the same directions as are described in #1 but use the acid and alcohol listed below in place of the two used in # 1. (a) Carboxylic acid acetic acid Alcohol isoamyl alcohol H3C O H3C OH (b) butyric acid C H2 H3 C (c) H3C OH butyric acid H3 C OH (d) salicylic acid benzyl butyrate H2 C H C C C H2 OH benzyl alcohol O H2 C ethyl butyrate H2 C C C H2 OH ethyl alcohol O H2 C H2 C CH C H3C Ester Product isoamyl acetate HC C OH HC CH C H methyl alcohol methyl salicylate O H C HC C C HC OH H3C OH C C H OH Exp. 8 Page 2