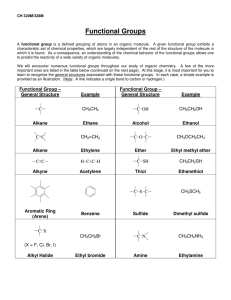
CHM 112 Spring 2007 WORKSHEET FOR ALCOHOLS, PHENOLS, AND ETHERS
advertisement

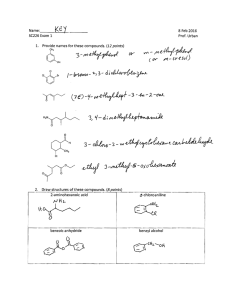
CHM 112 Spring 2007 WORKSHEET FOR ALCOHOLS, PHENOLS, AND ETHERS CH 3 1. Write the IUPAC name for the following compounds: H2 C CH H2 C H2 C CH H3C H3C CH 3 CH 3 CH CH CH 3 HO C CH 2 CH 3 CH CH 3 OH OH CH 3 2, 4-dimethyl-2-hexanol cyclohexanol Br 6-methyl-4-isopropyl-2-heptanol H2 C HO C H2 OH 3-bromophenol m-bromophenol OH H2 C 1, 2-ethandiol ethylene glycol CH CH H3C CH 3 C H2 OH CH 3 4-phenyl-3-hexanol H2 C CH H3C O CH 3 C H2 H2 C H3C isopropoxy propane isopropyl propyl ether 2. O ethoxy benzene ethyl phenyl ether Draw the full or condensed structural formulas for each of the following compounds: 3, 4-dimethyl-3-heptanol methoxy propane CH 3 H2 C H3C CH 3 H2 C CH C C H2 H2 C O H3C CH 3 3-ethyl-1-cyclopentanol C H2 CH 3 OH H3C C H2 OH 2-iodophenol cyclohexoxy octane H2 C H3C C H2 H2 C C H2 HO H2 C C H2 1, 3-propanediol O C H2 H2C CH 2 I H2C OH OH 3. Supply the appropriate reactant(s) or product(s) for the following reactions: H2O/H+ H C H3C CH CH H3C C H HO or H2 C H3C C H2 OH H2 C H3C H2 C H2 C CH CH 3 K2Cr2O7 H3C H2 C C CH3 C H2 C H2 O OH H2 C HO CH 3 CH CH 3 __________________________________ H C [O] O CH 3 CH CH 3 4. Explain why ethers generally have lower melting and boiling points than corresponding alcohols with the same molar masses. While ethers contain an oxygen, they do not have hydrogens attached to an oxygen. As a result, they are not able to hydrogen bond with other ether molecules. Since the forces between the ether molecules are weak dispersion forces, it does not take much energy to separate them from each other and convert from solid to liquid or liquid to gas. Alcohols can hydrogen bond and so more energy is required to separate molecules and the boiling point or melting point goes up.