CHM130 Galvanic (Voltaic) Cells Experiment: Voltaic

CHM130 Galvanic (Voltaic) Cells
Experiment: Voltaic Cells and Batteries
Introduction : An electrochemical reaction is a chemical reaction that involves reduction and oxidation (a redox reaction). The energy released in a spontaneous redox reaction can be used to do electrical work. This is accomplished using a voltaic (or galvanic) cell, a device in which the transfer of electrons occurs through an external pathway rather than directly between reactants.
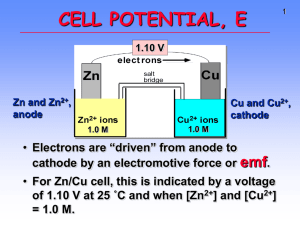
A spontaneous redox reaction takes place when a strip of zinc is put into a solution containing copper sulfate. The reaction is Zn(s) + Cu
2+ (aq) → Zn 2+
(aq) + Cu(s). Carrying out the reaction in this way, however, will not permit the obtaining of useful electrical energy. Instead, a zinc strip is placed into a solution of zinc sulfate in one container. A copper strip is placed into a solution of copper sulfate in another container. An electrical conducting wire is connected between the zinc and copper strips. A salt bridge containing an electrolyte capable of maintaining charge balance in each solution is placed between the two solutions. A voltmeter can be placed in the circuit to measure the potential generated in the reaction. Zinc is oxidized to
Zn
2+
in one compartment. The zinc strip is called the anode electrode. Cu
2+
is reduced to copper in the other compartment. The copper strip is called the cathode electrode. The electrons lost by the zinc to form Zn
2+
travel from the anode through the external circuit to the cathode and are picked up by Cu
2+
ions as they form copper atoms on the electrode. A diagram of this voltaic (or galvanic) cell is shown in the figure below:
The electrochemical reaction can be represented as two half-reactions:
Zn(s) → Zn 2+
(aq) + 2e
-
anode
Cu
2+
(aq) + 2e
-
→ Cu(s) cathode
Zn(s) + Cu
2+ (aq) → Zn 2+
(aq) + Cu(s) overall reaction
If all the components of the cell are in their standard states (with [Zn
2+
] = 1.0 M and [Cu
2+
] = 1.0
M), the cell is called a standard cell. The voltage generated by the standard cell is called the standard cell potential (E ). The shorthand notation for this cell is as follows:
Zn(s) | Zn
2+
(1.0M) | | Cu
2+
(1.0M) | Cu(s)
Page 2 of 3
In this designation the anode or oxidation half-cell is represented on the left side and the cathode or reduction half-cell is represented on the right side. The electrodes in the two half-reactions are electrically connected using a salt bridge represented by two vertical lines. The cell electrodes are represented at the two ends in this notation. A single vertical line shows the phase boundary between the solid electrode and the electrolyte solution in each half-cell.
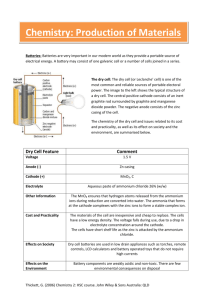
Modern battery technology takes advantage of oxidation/reduction reactions to manufacture the numerous types of batteries in devices that are used every day. Common battery chemistries include:
Zinc-carbon battery: The zinc-carbon chemistry is common in many inexpensive AAA,
AA, C and D dry cell batteries. The anode is zinc
, the cathode is manganese dioxide, and the electrolyte is ammonium chloride or zinc chloride.
Alkaline battery: This chemistry is also common in AA, C and D dry cell batteries. The cathode is composed of a manganese dioxide mixture, while the anode is a zinc powder.
It gets its name from the potassium hydroxide electrolyte, which is an alkaline substance.
Lithium-ion battery (rechargeable) : Lithium chemistry is often used in high-performance devices, such as cell phones, digital cameras and even electric cars. A variety of substances are used in lithium batteries, but a common combination is a lithium cobalt oxide cathode and a carbon anode.
Lead-acid battery (rechargeable) : This is the chemistry used in a typical car battery. The electrodes are usually made of lead dioxide and metallic lead, while the electrolyte is a sulfuric acid solution.
Materials:
This exercise will require the use of the following glassware and hardware:
1.
Volt meter
2.
Beaker with connected salt bridge (10)
3.
Alligator clips
This exercise will use the following chemicals:
1.
Metal electrodes: Cu, Zn, Sn, Pb, Fe
2.
Solutions of metal ions: Cu
2+
, Fe
2+
, Pb
2+
, Zn
2+
, Sn
2+
with a concentration of 0.1 M
Page 3 of 3
Procedure: This exercise contains two parts, measuring EMF of common batteries and measuring various electrochemical cell potentials. Each part has separate pages of instructions to enter collected data and make calculations. The Excel spreadsheet should be down loaded to the laboratory computer and opened from its hard drive during the experiment.
Part One – Batteries
Various batteries are distributed on the center lab benches. Measure the EMF of the cell or combination of cells and record the voltage in the spreadsheet.
Part Two – Voltaic (Galvanic) Cells
Various electrochemical cells will be assembled and their voltages measured. The cells will be assembled with small beakers and a salt bridge. Approximately 40 ml of Cu
2+
, Fe
2+
, Pb
2+
,
Zn
2+
, Sn
2+
of each solution is contained in the beakers. The metal electrode should match the solution that it is in. The voltage will be measured with a volt meter and alligator clips to the electrodes. Black wire should be on the left and the Red wire should be on the right.
Record the voltages and polarity of all the possible combinations. Note: some of the numbers will be negative; do not reverse the black and red wires.
Based on the information collected, rate the five metals with respect to their reactivity or activity. The rating is done based on the values of the potentials collected and which metal is the anode, and which the cathode in the ten cell combinations.
For each cell,
1.
Draw a picture of each galvanic cell labeling all of the parts (electrode metal, solution contents, salt bridge, anode, cathode, black or red wire, direction of electrons).
2.
Write the shorthand notation for the cell. Note: the solutions are all 0.1M and the ions are all +2.
3.
Write the half reactions for each cell and the overall balanced chemical equation
(combination of the two half reactions).