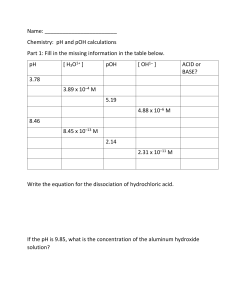
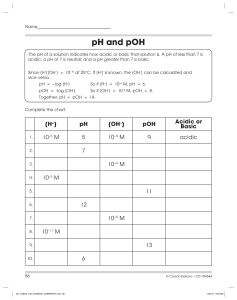
Name: :______ The following problems will help you prepare for
advertisement

Name:______________________________________________Per:__________ The following problems will help you prepare for your quiz next time on this topic. Check the key and get stamped when finished. This page goes on pg. 104 in your notebook. Formulas: pH = - log [H+] pOH = - log [OH-] 14 = pH + pOH [H+] = 10^-pH [OH-] = 10^-pOH Problems: 1. Determine the pH and pOH of each of the following solutions and indicate whether each is acidic, basic, or neutral. Solution a) Milk, [H+] = 3.2 x 10-7 M b) Pickle Juice, [H+] = 2.0 x 10-4 M c) Beer, [H+] = 3.2 x 10-5 M d) Blood, [H+] = 4.0 x 10-8 M e) Seawater, [H+] = 3.0 x 10-9 M pH pOH Acid/Base/Neutral 2. Determine the [H+] and [OH-] of each of the following solutions, and indicate whether each is acidic, basic, or neutral. Substance [H+] [OH-] a) Lime Juice, pH = 1.9 b) Tomato juice, pH = 4.2 c) Saliva, pH = 7.0 d) Kitchen cleanser, pH = 9.3 e) Milk of Magnesia, pH = 10.50 f) Household Ammonia, pH = 11.90 Write the equation for the following reactions involving acids and bases 3. Hydrochloric acid (HCl) and sodium hydroxide (NaOH) 4. Sulfuric acid (H2SO4) and calcium hydroxide (Ca(OH)2) Acid/Base/ Neutral