Quiz3.docx
advertisement

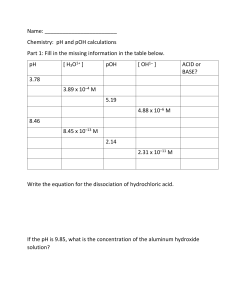
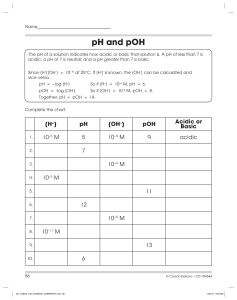
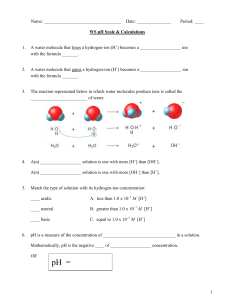
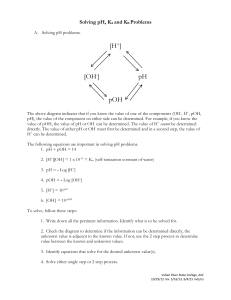
Name ______________________ CHEM 1474 Quiz #3 Fall 2010 (Buckley) 1. (5 points) Consider each of the following systems to be initially at equilibrium. The indicated stress is applied. State whether the equilibrium would shift to the left, to the right, or not shift in response to the applied stress. Shift? Applied Stress (Left, right, or none) 2 SO2 ( g ) O2 ( g ) 2 SO3 ( g ) 4 HBr ( g ) O2 ( g ) 2 H 2O( ) 2 Br2 ( ) N 2 ( g ) 3H 2 ( g ) H 2CO3 (aq ) Reduce volume H 426 kJ 2 NH 3 ( g ) Add catalyst H 2O( ) CO2 ( g ) Ag (aq) 2 NH 3 (aq) Heat the vessel Increase volume Ag ( NH 3 ) 2 ( aq) NaCl is added 2. (3 points) Write the formula for the conjugate acid of each of the following. Be sure to include the charge if an ion is formed. a. CH3NH2 ________________ b. HCO3- ________________ c. H2O ________________ 3. (3 points) An aqueous solution has a pH of 6.75. Find the pOH, [H+], and [OH-] for this solution. Show your work. 4. (7 points) Find the pH, pOH, [H+], and [OH-] for a 0.170 M solution of HCN. The Ka for HCN is 4.9 × 10-10. Show your work. 5. (7 points) Find the pH, pOH, [H+], and [OH-] for a 0.35 M solution of C5H5N. The Kb for C5H5N is 1.7 × 10-9. Show your work.