Acid Base Virtual Lab
advertisement

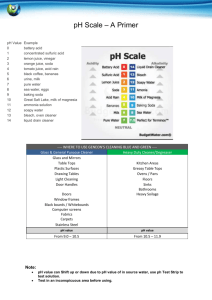
Name ___________________________________ Date________________ Acid/Base Virtual Lab Go to: http://www.glencoe.com/sites/common_assets/science/virtual_labs/E22/E22.html Read the introduction and answer the following questions: 1. What does the pH of a solution measure? 2. How does pH paper help to determine the pH? 3. What is the range of the pH scale? 4. What is the pH of an acid? 5. What is the pH of a base? 6. What is the pH of a neutral substance? Write down the pH and the color of the paper for the following substances you tested. Decide if the substance is acidic/basic or neutral. Substance pH Color Acid/Base/Neutral 1. Battery acid _________________________________________________________ 2. Oven Cleaner _________________________________________________________ 3. Lemon juice _________________________________________________________ 4. Shampoo _________________________________________________________ 5. Vinegar _________________________________________________________ 6. Sea Water _________________________________________________________ 7. Soft drink _________________________________________________________ 8. Antacid _________________________________________________________ 9. _________________________________________________________ Tomatoes 10. Stomach Acid _________________________________________________________ 11. Orange Juice _________________________________________________________ 12. Pure Water _________________________________________________________ 13. Antacid _________________________________________________________ Go to phet: ph Scale http://phet.colorado.edu/sims/html/ph-scale/latest/ph-scale_en.html Click on the tab labeled, “Micro” Experiment with each solution. Notice what happens to the bar graph as the pH changes. Record the following: pH [H3O+] [OH-] 1. Drain cleaner _____________________________________________________________ 2. Hand soap ___________________________________________________________ 3. blood __________________________________________________________ 4. spit __________________________________________________________ 5. water __________________________________________________________ 6. milk __________________________________________________________ 7. coffee __________________________________________________________ 8. beer __________________________________________________________ 9. soda pop __________________________________________________________ 10.vomit __________________________________________________________ 11. battery acid __________________________________________________________ Make a custom liquid and write down the following: pH [H3O+] [OH-] 1. What is the relationship between the concentration of the hydronium and hydroxide ion and pH in any water solution? 2. If a solution is neutral, how many hydronium ions are present? 3. If a solution is neutral, how many hydroxide ions are present?