Acids and Bases Overview
advertisement

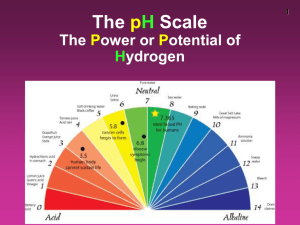
Name:_____________________________________ Date:___________________ Per:____ Acids and Bases Review Directions: Complete the following. Include units where necessary. Place your answers in the answer sheet provided. [H+] = 1 x 10-14 M2 or [OH-] = 1 x 10-14 M2 [OH-] [H+] pH + pOH = 14 pH = -log [H+] [H+] = 10 ^ - pH 1. A solution has a hydroxide ion concentration of 2.6 x 10 -11 M. What is the pH for the solution? 2. What is the [H+] of a solution that has a pOH of 9? 3. Solve for pOH if pH is 2. 4.What is the pH of a solution if [H+] = 3.50 x 10-9 M? 5.A solution has [H+] = 1.00 x 10 -7 M. Is it an acid, base, or neutral? 6. A solution has pH = 1.5. Is it an acid, base, or neutral? 7. An acidic solution would have a higher/lower concentration of hydrogen ions? 8. A solution has [H+] = 4.56 x 10-11 M. What is [OH-]? 9. A solution has a pOH = 14. Is it an acid, base, or neutral? 10. A substance is said to be _____________________ if it has a pH of exactly 7. 11. ________________________ turn red litmus paper blue, taste bitter, and feel slippery. 12. ________________________ turn blue litmus paper red and taste sour. 13. _______________________ is the measure of the acidity or alkalinity of a solution. 14. The pH of a basic solution is ________________ than 7. 15. For two acids of equal concentration, the stronger acid has the (higher/lower) _____________ concentration of hydronium (H3O+) ions. 16. Sea water is an example of a strong ___________________ because it conducts electricity. Name:_____________________________________ Date:___________________ Per:____ Acids and Bases Review Directions: Complete the following. Include units where necessary. Place your answers in the answer sheet provided. [H+] = 1 x 10-14 M2 or [OH-] = 1 x 10-14 M2 [OH-] [H+] pH + pOH = 14 pH = -log [H+] [H+] = 10 ^ - pH 1. A solution has a hydroxide ion concentration of 2.6 x 10 -11 M. What is the pH for the solution? 2. What is the [H+] of a solution that has a pOH of 9? 3. Solve for pOH if pH is 2. 4.What is the pH of a solution if [H+] = 3.50 x 10-9 M? 5.A solution has [H+] = 1.00 x 10 -7 M. Is it an acid, base, or neutral? 6. A solution has pH = 1.5. Is it an acid, base, or neutral? 7. An acidic solution would have a higher/lower concentration of hydrogen ions? 8. A solution has [H+] = 4.56 x 10-11 M. What is [OH-]? 9. A solution has a pOH = 14. Is it an acid, base, or neutral? 10. A substance is said to be _____________________ if it has a pH of exactly 7. 11. ________________________ turn red litmus paper blue, taste bitter, and feel slippery. 12. ________________________ turn blue litmus paper red and taste sour. 13. _______________________ is the measure of the acidity or alkalinity of a solution. 14. The pH of a basic solution is ________________ than 7. 15. For two acids of equal concentration, the stronger acid has the (higher/lower) _____________ concentration of hydronium (H3O+) ions. 16. Sea water is an example of a strong ___________________ because it conducts electricity.