AP Biology Biochemistry (Water)
advertisement

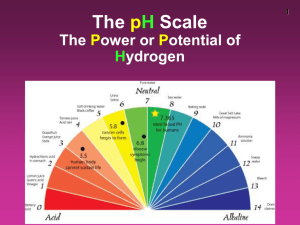
AP Biology Biochemistry (Water) – Part 2 (Associated Learning Objectives: 4.1, 4.2, 4.3, 4.14, 4.15, 4.17) I. Water is the Universal Solvent A. Solvent – Liquid that is doing the dissolving of another substance. B. Solute – Substance being dissolved in the solute. C. Solution – Substance possessing equal distribution of material. (Kool-aid is a good example.) D. Suspension – Temporary suspension of material. (Italian Dressing herbs in a bottle are a good example.) E. Colloid – Extended temporary suspension of material. (Milk is a good example.) F. HYDROGEN bonds of water make each situation possible. G. Hydration shell – Water surrounding a molecule. Substance is dissolved and “disappears”. H. Oils, grease, and fat are NON-POLAR and therefore water can’t grab and dissolve. (Need salt to make a molecular bridge to dissolve… MOST dishwashing liquids are just SALTWATER with coloring.) II. Hydrophobic “hydro” means water; “phobic” means fear of A. Water cannot attach to the substance because the substance is non-polar. B. The substance “hates” water’s polarity. III. Hydrophilic “philic” means love of A. Water can attach to the substance because the substance is polar. B. The substance “loves” water’s polarity. IV. “WET” Chemistry Terminology A. Mole (mol) 1. Refers to a measurement of molecules that is relative to its molecular weight. 2. Avogadro’s Number 6.02 x 10²³ a. # of molecules of that particular substance present in a 1 mole. 3. Find the molecular weight of a molecule using the Periodic Table and then weigh out that many grams of the substance and that amount is equal to 1 mole. (Sucrose = 342 so I need 342 grams) B. Molarity 1. Term for telling how many moles of a substance are dissolved in a solution. (usually water) C. Dissociation 1. Refers to water breaking apart into H+ (Proton) and an OH- (hydroxide Ion). 2. Acid – a substance that gives away H+. (Measured on a pH scale.) a. Scale goes from 0 to 14. b. 7 neutral c. ON THE pH SCALE:<7- substance is an ACID; >7 – Substance is a BASE 3. Base – a substance that gives away OH-. (Measured on a pOH scale.) a. ON THE pOH SCALE: <7 –Substance is a BASE; >7 – Substance is an ACID 4. The scales are inverse. D. Buffer 1. A substance that can resist changes in pH or pOH. 2. It can take on or gives off a H+ or OH- to maintain the pH or pOH concentration. 3. Good example is Human Blood –The buffer is Bicarbonate ( HCO₃¯ ). a. Bicarbonate helps keeps blood at a pH of 7.4 ideally b. It is needed because of the food, drink, air or other substances we put into our bodies c. Blood pH is below 7.2 (This condition is called Acidosis and can be deadly.) d. Blood pH is above 7.6 (This condition is called Alkalosis and can also be deadly.) e. “sis” means “the condition of being” 4. The pH of the blood affects oxygen’s ability to adhere to red blood cells. E. Acid Precipitation (Refers to Rain, Snow, Sleet, Ice, or Fog with a low pH.) 1. Water falling in the environment that has a pH of less than 5.6. 2. Mainly because of SO (Sulfur Oxide) and NO (Nitrous Oxide) in the air to combine with water. a. Both are found in fossil fuels when burned. (Such as oil, gasoline, or diesel fuel) 3. Leaching a. This refers to the rain pushing nutrients away from plant roots to deeper in the soil. b. Process causes the plant to starve and the rain burns the plants leaves. 4. Lowest was about 3.2 in Ohio, West Virginia, and Pennsylvania. a. During the Mid 1970’s. b. This brought about massive regulation laws for industry.