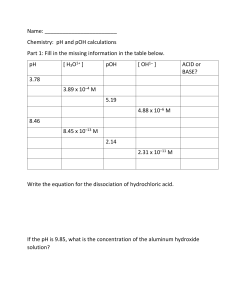
pH & pOH Calculation WS pH Calculation: Kw = [H+][ OH-] = 1.00 x 10-14 pH = -Log[H+] OR [H+] = 10-pH pOH = -Log[OH-] OR [OH-] = 10-pOH pH + pOH = pKw = 14.000 Titration: MaVana = MbVbnb Direction: Show all work for each problem. 1. a) Calculate the concentration and 50.0 mL of HCl if 18.3 mL of standardized 0.00100 M NaOH is used in a neutralization titration. b) Calculate the pH and pOH of the unknown HCl from part (a). 2. a) What volume of 0.0045 M NaOH is needed to neutralize 50.0 mL of 0.000750 M HCl in a neutralization reaction? b) Calculate the pH and pOH of 0.0045 M NaOH from part (a). 3. Solve and write the final answer in the chart below: SHOW WORK for each box on the right side!!! Question [H+] pH pOH [OH–] a 1.0 x10-4 b 3.9 c 1.2 d 8.3 x10-8