CHEMISTRY 12 pH WORKSHEET
advertisement

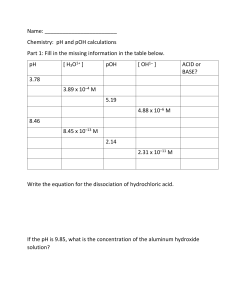
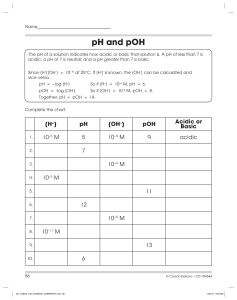
CHEMISTRY 12 pH WORKSHEET Name: ________________________________ Date: ______________ Block: ______________ 1. 2. 3. Calculate pH and pOH for the following solutions : a) [H+] = 1.0 x 10-5 M b) [OH-] = 3.0 x 10-8 M c) [H+] = 2.5 x 10-2 d) [OH-] = 7.5 x 10-3 M e) [H+] = 1.2 x 10-14 M f) [H+] = 6.0 M Calculate [H+] and [OH-] for the following : a) pH = 3.0 b) pOH = 2.60 c) pOH = 5.63 d) pH = 7.51 e) pOH = -1.13 f) pH = 0.03 Calculate the pH and the pOH of 0.050 M solutions of the following acids : a) Perchloric acid, HClO4 b) Carbonic acid, H2CO3 c) Phenol, C6H5OH d) Aluminum ion, Al(H2O)63+ 533582277 4. Boric acid (H3BO3) is commonly used in eyewash solutions in chemistry laboratories to neutralize bases splashed in the eye. It acts as a monoprotic acid, but the dissociation reaction is slightly different from that of other acids: B(OH)3 + H2O < === > B(OH)4- + H+ Ka= 5.8 x 10-10 Calculate the pH of a 0.50 M solution of boric acid. 5. Calculate the pH of the solution formed when 35.0 mL of 1.00 M HCl is mixed with 175.0 mL of 0.25M NaOH. 6. Solution A is prepared with a pH of 2.50, and solution B is prepared with a pH of 5.00. How many times more acidic is solution A than solution B. Support your answer with calculations. ANSWERS : 1. pH a) 5.00 b) 6.48 c) 1.60 d) 11.88 e) 13.92 f) -0.78 3. a) b) c) d) g) pOH 9.00 7.52 12.40 2.12 0.08 14.78 1.30, 12.70 3.83, 11.17 5.59, 8.41 3.08, 10.92 4.15, 9.85 533582277 2. a) b) c) d) e) f) 4. 5. 6. [H+] 1 x 10-3 M 4.0 x 10-12 M 4.3 x 10-9 M 3.1 x 10-8 M 7.4 x 10-16 M 0.93 M 4.77 12.62 316 x [OH-] 1 x 10-11 M 2.5 x 10-3 M 2.3 x 10-6 M 3.2 x 10-7 M 13 M 1.1 x 10-14 M