Assignment: Elements Name:
advertisement

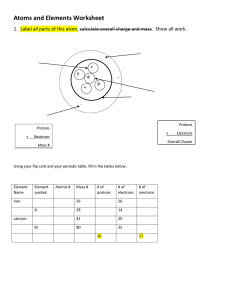
Assignment: Elements Name: Considering what you learned in at the graphite and diamond site, describe the differences between the two. Which 4 elements make up most living things? Go to the following websites and answer the following questions: http://education.jlab.org/indexpages/index.html - The Homework Helpers links will be particularly helpful including All about Atoms, Table of Elements, Questions and Answers and Glossary of Science Terms. http://www.ndt-ed.org/EducationResources/HighSchool/Radiography/atomselements.htm (You can also do some additional research on your own.) I. Matter and Energy a) Everything in the universe is made of two things: _________________ and ____________________ b) Atoms are particles of elements, ______________________________________________________ c) Compound: ________________________________________________________________________ d) The difference between states of matter is due to __________________________________________ f) What keeps the electron and proton from crashing into each other? What is a good example of this? (In your own words) g) Describe how far away an electron orbits from the nucleus in your own words h) It is not accurate to show an electron spinning around the nucleus like a ball in a circle because . . . i) Atomic Number = ____________________________________________________________________ j) Atomic Mass = ______________________________________________________________________ Directions: 1. Explore the Build an Atom simulation. http://phet.colorado.edu/en/simulation/build-an-atom 2. Using Build an Atom, play with the parts of atoms to find about Atomic Structure 3. a) Atoms are made of 3 different particles (include description): i) ii) iii) A. What parts go in the center of the atom? What is the center called? B. Play until you discover a good rule for making the center of the atom “stable”. What seems to make the center of the atom “unstable”? C. Make a table like the one below to identify three examples – at least 1 stable and at least 1 unstable – that shows your rules for stablility work and include a drawing of your nucleus. What is in your nucleus? Draw your nucleus Is it stable or unstable? What Element is it? 1 2 3 3. Everything around us is made up of different elements. The air has Oxygen and Nitrogen. Plants and people have lots of Carbon. Helium is in balloons. Hydrogen is in water. A. Play until you discover a rule for what determines the name of the element you build. What did you find determines the element? B. Test your idea by identifying the element for the following 3 cases: example 1 2 3 Atom or Ion has What Element is it? # of protons: 6 # of neutrons: 6 # of electrons: 6 # of protons: 7 # of neutrons: 6 # of electrons: 6 # of protons: 6 # of neutrons: 7 # of electrons: 7 4. Play until you discover some good rules about the charge of your atom or ion. What is a rule for making: 1) A neutral atom which has no charge. 2) A positive ion which has positive charge? 3) A negative ion which has negative charge? Practice – use the information given to complete the chart. Element Oxygen Gold # protons 8 79 # electrons 8 79 # neutrons 8 118 Atomic Number 8 79 Atomic Mass 16 197 Potassium Sulfur Hydrogen Iron Iodine Uranium Krypton Silicon Erbium 19 16 20 26 53 0 30 16 1 127 92 146 14 28 36 68 39 32 1 83 167