Document

Hypothetical substrate docking in enzyme’s active site . Substrate is geometrically and electronically compatible with active site.
Enzymes are also stereospecific .
ES complex formation
How is
G ‡
lowered?
Many enzymes stabilize the transition state in addition to the mechanisms described below through relatively tight binding.
Mechanisms of catalysis
1. Acid-Base catalysis
2. Covalent catalysis
3. Metal ion catalysis
4. Proximity and orientation effects
5. Electrostatic catalysis
1. Acid-Base catalysis (H + transfer between E and S)
Acid catalysis
Base catalysis
2. Covalent catalysis (covalent bond between E and S)
Amine in enzyme reacts with carbonyl group to form Schiff base
Elimination of CO
2 then does not lead to an unstable transition state
Schiff base breaks down to amine catalyst and acetone
3. Metal ion catalysis (metal ions can mediate redox reactions or electrostatically promote reactions).
Proximity/Orientation
No catalyst: slow b/c few encounters
Catalyst: faster
5. Electrostatic catalysis
Most substrate molecules have a hydration shell that must be removed to allow reaction.
Enzyme active sites are often non-aqueous (not necessarily non-polar) and they require that water is removed from the substrate before binding can occur.
The non-aqueous active site allows stronger electrostatic interactions.
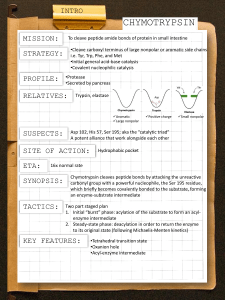
Chymotrypsin
Digestive enzyme synthesized in pancreas and secreted to small intestine.
Has broader specificity than most enzymes.
Hydrolyzes the peptide bond following large nonpolar residues like Phenylalanine, Tryptophan or
Tyrosine.
Leu-AlaTyr -Ile-Asp
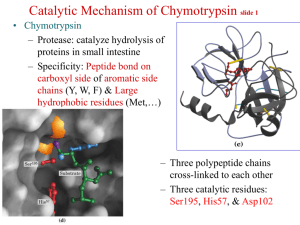
Chymotrypsin. 241 amino acid protein with 2 domains and an active site with 3 residues important for catalysis shown in red (His 57, Asp
102 and Ser 195).
Chymotrypsin is part of a class of enzymes known as
Serine Proteases.
Uses acid-base and covalent catalysis, and it stabilizes the transition state.
His 57
Asp 102
Ser 195
Let’s look at the detailed reaction mechanism for chymotrypsin, a protease:
It hydrolyzes peptide bonds immediately
C-terminal to aromatic amino acids
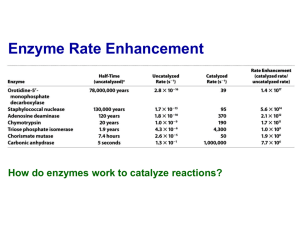
With a rate enhancement of > 10 9
And illustrates several principles
Transition state stabilization
Acid-base catalysis
Covalent catalysis
A two-phase reaction
Leu-AlaTyr -Ile-Asp
16
Appreciate the contribution of protein folding
PDB: 7GCH
17
Mechanism in the
Substrate Pocket
• Phase I - acylation of
Ser 195
• Phase 2 – deacylation of
Ser 195
• Follow the roles of the
“catalytic triad” through the reaction: Ser 195 , His 57 and Asp 102
• What is the role of
Gly 193 ?
18
In the active site of chymotrypsin, know the chemical roles of:
• Histidine 57
• Aspartate 102
• Serine 195
• Glycine 193
19
Chymotrypsin Mechanism -1
20
Chymotrypsin Mechanism -2
21
Chymotrypsin Mechanism - 3
22
Chymotrypsin Mechanism - 4
23
Chymotrypsin Mechanism - 5
24
Chymotrypsin Mechanism - 6
25
Chymotrypsin Mechanism - 7
26
Chymotrypsin Catalytic Mechanism A1
Catalytic Triad
His57
Asp102
H
H
Ser195
O
[ HOOC ]
N
C
C
N
H C
O
C
N
C
C
[ NH
2
]
Check substrate specificity
Chymotrypsin Catalytic Mechanism A2
Asp102
H
His57
H
Ser195
O
First Transition State
Chymotrypsin Catalytic Mechanism A3
H
H
O
Acyl-Enzyme Intermediate
Chymotrypsin Catalytic Mechanism D1
H
O
Acyl-Enzyme Water Intermediate
Chymotrypsin Catalytic Mechanism D2
H
H
O
O
Second Transition State
Chymotrypsin Catalytic Mechanism D3
H
H
O
O
Deacylation