Lecture 9
advertisement

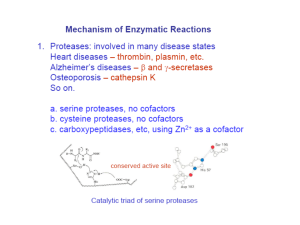
Lecture 17 – Exams in Chemistry office with M’Lis. Please show your ID to her to pick up your exam. – Quiz on Friday – Enzyme mechanisms Terms to review for enzymes • • • • • • • • • Cofactor Coenzyme Prosthetic group Holoenzyme Apoenzyme Lock and Key Transition analog model Induced fit Active site, binding site, recognition site, catalytic site Catalytic Mechanisms • Acid-base catalysis • Covalent catalysis • Metal ion catalysis • Proximity and orientation effects (ex. anhydride) • Preferential binding of the transition state complex General Acid-Base Catalysis • Large number of possible amino acids • Requires that they can accept and donate a proton • Glu, Asp • Lys, His, Arg • Cys, Ser, Thr • Also can include metal cofactors (metal ion catalysis) • Example can be observed in RNAse Page 499 Figure 15-2 The pH dependence of V¢max/K¢M in the RNase A–catalyzed hydrolysis of cytidine-2¢,3¢ -cyclic phosphate. Example in book: RNAse (p. 499) RNAse mechanism •His12 acts as general base-takes proton from RNA 2’-OH-making a nucleophile which attacks the phosphate group. •His119 acts as a general acid to promote bond scission. Page 499 •2’,3’ cyclic intermediate is hydrolyzed through the reverse of the first step-water replaces the leaving group. His12 is the acid, His119 acts as the base Covalent catalysis • Rate acceleration through the transient formation of a catalyst-substrate covalent bond. • Example-decarboxylation of acetoacetate by primary amines • Amine nucleophilically attacks carbonyl group of acetoacetate to form a Schiff base (imine bound) Figure 15-4 The decarboxylation of acetoacetate. Page 500 uncatalyzed e- sink Catalyzed by primary amine Covalent catalysis • Made up of three stages 1. The nucleophilic reaction between the catalyst and the substrate to form a covalent bond. 2. The withdrawal of electrons from the reaction center by the now electrophilic catalyst 3. The elimination of the catalyst (reverse of 1.) • Nucleophilic catalysis - covalent bond formation is limiting. • Electrophilic catalysis-withdrawal of electrons is rate limiting Covalent catalysis • Nucleophilicity is related to basicity. Instead of abstracting a proton, nucleophilically attacks to make covalent bond. • Good covalent catalysts must have high nucleophilicity and ability to form a good leaving group. • Polarized groups (highly mobile e-) are good covalent catalysts: imidazole, thiols. • Lys, His, Cys, Asp, Ser • Coenzymes: thiamine pyrophosphate, pyridoxal phosphate. Covalent Catalysis • Form transient, metastable intermediates that can supply bond energy into the reaction. Examples structures Side chain Chymotrypsin NH O Trypsin Serine Elastase RC-O-CH -CH 2 (acyl ester) COO- Serine O NH -O-P-O-CH -CH 2 O (phosphoryl ester) COO- acetylcholinesterase Phosphoglucomutase Alkaline phosphatase Covalent Catalysis Group O Cysteine Examples structures NH Papain 3-PGAL-DH RC-S-CH2-CH (acyl cysteine) COO- Histidine O -O-P-N CH NH O (phosphoryl imidazole) COO- Succinate thiokinase Covalent Catalysis Group R' Lysine Examples structures NH R-C=N-(CH2)4-CH (Schiff base) COO- Aldolase Transaldolase Metal ion catalysis • Almost 1/3 of all enzymes use metal ions for catalytic activity. 2 main types: 1. Metalloenzymes-have tightly bound metal ions, mmost commonly transition metal ions such as Fe2+, Fe3+, Cu2+, Zn2+, Mn2+, or Co3+ 2. Metal-activated enzymes-loosely bind metal ions form solution-usually alkali or alkaline earth metals-Na+, K+, Ca2+ Metal ion catalysis • Three ways for catalysis 1. Binding to substrates to orient them properly for the reaction 2. Mediating oxidation-reduction reactions through reversible changes in the metal ion’s oxidation state 3. Electrostatically stabilizing or shielding negative charges. Serine Hydrolases (Proteases) • Chymotrypsin, trypsin and elastase. • All have a reactive Ser necessary for activity. • Catalyze the hydrolysis of peptide (amide) bonds. • Chymotrypsin can act as an esterase as well as a protease. • Study of esterase activity provided insights into the catalytic mechanism. O CH3 C O NO2 p-Nitrophenylacetate Chymotrypsin H2O 2H+ O CH3 C Acetate O- + -O NO2 p-Nitrophenolate Serine Hydrolases (Proteases) • Reaction takes place in 2 phases 1. The “burst phase”-fast generation of pnitrophenolate in stoichiometric amounts with enzyme added 2. The “steady-state phase”-p-nitrophenolate generated at reduced but constant rate; independent of substrate concentration. Page 516 Figure 15-18 Time course of pnitrophenylacetate hydrolysis as catalyzed by two different concentrations of chymotrypsin. O CH3 C O NO2 + Enzyme Chymotrypsin p-Nitrophenylacetate -O FAST O CH3 C NO2 p-Nitrophenolate O-Enzyme Acyl-enzyme intermediate SLOW O H2O 2H+ CH3 C O- + Enzyme Acetate Chymotrypsin • Follows a ping pong bi bi mechanism. • Rate limiting step for ester hydrolysis is the deacylation step. • Rate limiting step for amide hydrolysis is first step (enzyme acylation). Identification of catalytic residues (active Ser)-CH2OH + • Identified catalytically important residues by chemical labeling studies. • Ser195-identified using diisopropylphosphofluoridate (DIPF) • Irreversible! CH(CH3)2 O F-P=O Diisopropylphospho -fluoridate (DIPF) O CH(CH3)2 CH(CH3)2 O (active Ser)-CH2O-P=O O CH(CH3)2 DIP-enzyme Identification of catalytic residues • His57 was identified through affinity labeling • Substrate analog with a reactive group that specifically binds to the active site of the enzyme forms a stable covalent bond with a nearby susceptible group. • Reactive substrate analogs are sometimes called “Trojan horses” of biochemistry. • Affinity labeled groups can be identified by peptide mapping. • For chymotrypsin, they used an analog to Phe. Identification of catalytic residues O CH3 S CH2 O NH CH C CH2Cl O Tosyl-L-phenylalanine chloromethyl ketone (TPCK) Page 517 Figure 15-19 Reaction of TPCK with chymotrypsin to alkylate His 57. Homology among enzymes • Bovine chymotrypsin, bovine trypsin and porcine elastase are highly homologous • ~40% identical over ~240 residues. • All enzymes have active Ser and catalytically essential His • X-ray structures closely related. • Asp102 buried in a solvent inaccessible pocket (third enzyme in the “catalytic triad”) X-ray structures explain differences in substrate specificity • Chymotrypsin - bulky aromatic side chains (Phe, Trp, Tyr) are preferred and fit into a hydrophobic binding pocket located near catalytic residues. • Trypsin - Residue corresponding to chymotrypsin Ser189 is Asp (anionic). The cationic side chains of Arg and Lys can form ion pairs with this residue. • Elastase - Hydrolyzes Ala, Gly and Val rich sequences. The specificity pocket is largely blocked by side chains of Val and a Thr residue that replace Gly residues that line the binding pocket of chymotrypsin and trypsin. X-Ray structure of bovine trypsin. (a) A drawing of the enzyme in complex. Page 518 Figure 15-20a Page 519 Figure 15-20b X-Ray structure of bovine trypsin. (b) A ribbon diagram of trypsin. Page 519 Figure 15-20c X-Ray structure of bovine trypsin. (c) A drawing showing the surface of trypsin (blue) superimposed on its polypeptide backbone (purple). Page 520 Figure 15-21 The active site residues of chymotrypsin. Page 521 Figure 15-22 Relative positions of the active site residues in subtilisin, chymotrypsin, serine carboxypeptidase II, and ClpP protease. Page 522 Figure 15-23 Catalytic mechanism of the serine proteases.