Chymotrypsin Mechanism Animation

INTRO
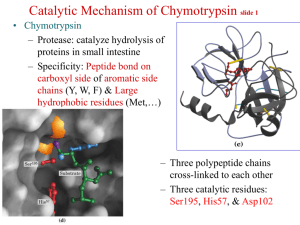
CHYMOTRYPSIN
MISSION:
To cleave peptide amide bonds of protein in small intestine
STRATEGY:
• Cleave carboxyl terminus of large nonpolar or aromatic side chains i.e. Tyr, Trp, Phe, and Met
• Initial general acid-base catalysis
• Covalent nucleophilic catalysis
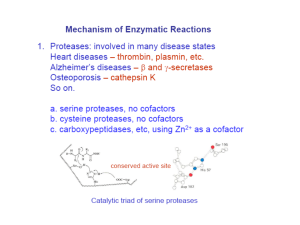
PROFILE: • Protease
• Secreted by pancreas
RELATIVES:
Trypsin, elastase
Aromatic
Large nonpolar
Positive charge Small nonpolar
SUSPECTS: Asp 102, His 57, Ser 195; aka the “catalytic triad”
A potent alliance that work alongside each other
SITE OF ACTION:
Hydrophobic pocket
ETA:
16x normal rate
SYNOPSIS:
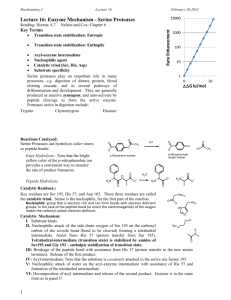
Chymotrypsin cleaves peptide bonds by attacking the unreactive carbonyl group with a powerful nucleophile, the Ser 195 residue, which briefly becomes covalently bonded to the substrate, forming an enzyme-substrate intermediate
TACTICS:
Two part staged plan
1. Initial “burst” phase: acylation of the substrate to form an acylenzyme intermediate
2. Steady-state phase: deacylation in order to return the enzyme to its original state (following Michaelis-Menten kinetics)
KEY FEATURES: • Tetrahedral transition state
• Oxanion hole
• Acyl-enzyme intermediate
TACTICS
CHYMOTRYPSIN
STAGE 1:
1
PREPARING FOR ATTACK
Acid base equilibria
Asp 102 attacks its comrade His 57 via H bonding which steals a H+ from Ser 195
Ser 195 is now ANGRY and a powerful NUCLEOPHILE
(an alkoxide anion)
TACTICS
CHYMOTRYPSIN
STAGE 1:
2
READY…SET…CHARGE!
Attack on the substrate
Powerful Ser 195 attacks the substrate where it hurts: at the weak carbonyl carbon, an easy target
A temporary tetrahedral transition state is formed, an unstable condition
Reinforcement: the oxyanion hole stabilizes the negative charge on the carbonyl
TACTICS
CHYMOTRYPSIN
STAGE 1:
3
RELIEVING THE TENSION
Transition state collapses
A stable acyl-enzyme intermediate is formed
The substrate carbonyl is attached to the Ser 195 side chain
Which is a covalent bond to the active site, hence this is known as “covalent catalysis”
TACTICS
CHYMOTRYPSIN
STAGE 1:
4
RETREAT! RETREAT!
Amino side of the substrate leaves the scene
See ya!
The remainder of the substrate, an amine product, leaves the active site
TACTICS
CHYMOTRYPSIN
STAGE 2:
FINALLY!
5
INTRUDER ALERT
Water enters the scene
Water enters the scene
His 57 interacts with water, causing it to be polarized
Water attacks the acyl-enzyme’s carbonyl in a nucleophilic attack ( “nucleophilic catalysis”)
TACTICS
CHYMOTRYPSIN
STAGE 2:
6
RELIEVING THE TENSION (AGAIN)
Transition state collapses
(again)
Another tetrahedral transition state is formed (again)
The oxyanion hole stabilizes the negative charge (again)
The tetrahedral transition state collapses (again)
(Notice the theme of “again”)
TACTICS
CHYMOTRYPSIN
STAGE 2:
7
RETREAT! RETREAT!(again)
The substrate leaves the scene
Adios!
The substrate, a carboxylate product, leaves the active site
Created by STEPHANIE LEUNG
Class of 2013
Note: the material covered on exams or in lecture may have changed, so I apologize if some of this is no longer relevant. Also, while much of this is my own work, the images and some of the text may have been copied from other sources. I do not claim it as my own. Lastly, I apologize if any of my material is incorrect/inaccurate. I’m just a student myself :) But please do let me know if there are any discrepancies so
I can correct them. Thanks!
Questions/comments: please contact me at sleung3@uic.edu