Test Objectives for Unit 13: Redox
advertisement

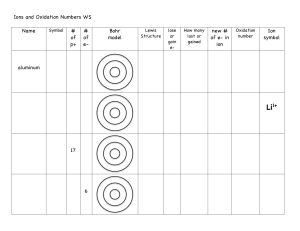
List the rules for assigning oxidation numbers: 1. 2. 3. 4. 5. the oxidation number of any free, uncombined elements is zero the oxidation number of any monatomic ion is the charge of the ion fluorine is always -1 hydrogen is nearly always +1, except when bonded to a metal element then hydrogen is -1 oxygen is nearly always -2 except: when bonded to fluorine oxygen is +2; when oxygen is used as a peroxide ion (O2 bonded to a group 1 or group 2 element) then oxygen is -1 6. the sum of all the oxidation numbers in a neutral compound is zero 7. the sum of all the oxidation numbers in a polyatomic ion is the charge of the ion 8. when non-metals only are bonded together in a compound or an ion the less electronegative element (+charge) is written first (exceptions where more electronegative element (-charge) is written first: NH3, NH4+1, OH-1) assign oxidation numbers to each element in the following compounds or polyatomic ions: +1 -1 1. K2S +3 +3 -2 6. Al2(C2O4)3 +1 +5 -2 11. Cs3PO4 +6 -2 16. SO3 +2 -1 2. CaBr2 +1 +3 -1 7. Ra(NO2)2 0 12. Ag +4 -2 17. SO3-2 +1 +6 -2 3. K2CrO4 +2 +5 -2 8. Ga(ClO3)2 +2 -1 13. HgBr2 +2 -1 18. CaH2 +2 +3 -2 4. Sr3(PO4)2 0 9. O2 +1 -2 14. ClO-1 -3 +1 19. NH4+1 +2 +5 -2 5. Mg(NO3)2 +3 -2 10. Al2O3 +5 -2 15. PO4-3 0 20. H2 NOTE: change to formula (C2O4)3