Notes: Oxidation Numbers
advertisement

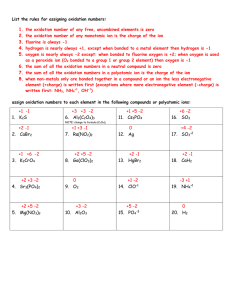
Notes: Oxidation Numbers Key Vocabulary Oxidation number – A number assigned to each of the atoms composing a compound or ion that is used to indicate the general distribution of electrons among the bonded atoms in a molecular compound or polyatomic ion. Oxidation state – Another name for oxidation number Oxidation numbers can help in understanding where electrons go when a compound is formed or when a reaction occurs. When an element or compound loses an electron it is said to have been oxidized. When an element or compound gains and electron it is said to have been reduced. LEO goes GER L Lose G Gain E Electron E Electron O Oxidized R Reduced An element or compound that causes another to lose electrons is said to be the oxidizing agent. An element or compound that causes another to gain electrons is said to be the reducing agent. The Rules Where the electrons are for polyatomic ions or molecular compounds is determined by the general rule of whichever atom is more electronegative gets the electron. More specifically: 1. Pure elements have an oxidation number of zero. (Na, O2, P4) 2. In a binary molecular compound the more electronegative element equals the charge it would have if an anion. The less electronegative elements is equal to the charge it would have if a cation. 3. Fluorine is always -1 4. Oxygen is usually equal to -2. Except when in a peroxide such as H2O2 then it is -1, OR when bonded to fluorine then it is +2. 5. Hydrogen is +1 when bonded to a nonmetal and is a -1 when bonded to a metal 6. The sum of oxidation numbers in a neutral compound is zero. 7. The sum of oxidation numbers in an ion equals the charge on the ion. Examples: HCl – Hydrogen is bonded to a nonmetal so it is +1 (rule 5) - since this is overall neutral, Cl must be -1 (rule 6) CF4 - Fluorine is always -1 (rule 3) - multiply by 4 atoms means F = -4 - since this is overall neutral, C must be +4 HClO3 - Hydrogen is bonded to a nonmetal so it is +1 (rule 5) - Oxygen is -2 (rule 4) - Multiply by 3 atoms means O = -6 - Since this is overall neutral Cl must equal +5 (rule 6) Oxidation Numbers and Formulas and Names Some nonmetals have more than one oxidation number and so can bond in different ratios with other elements to form different compounds (p 863 Appendix table A-15) This can be used to determine if compounds are possible and what the names of compounds would be. It can also be applied to the stock system. SO2 Sulfur Dioxide or Sulfur (IV) oxide SO3 Sulfur Trioxide or Sulfur (VI) oxide Resources Modern Chemistry text (Holt, Rinehart, and Winston) Page 232 – 235 and appendix page 863 Kahn Academy https://www.khanacademy.org/science/chemistry/oxidationreduction/v/redox-reactions