Chemical Formulas and Chemical Compounds Oxidation Numbers
advertisement

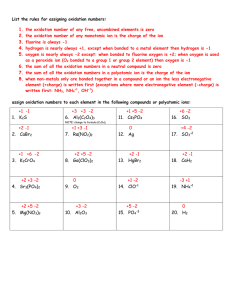
Chemical Formulas and Chemical Compounds Oxidation Numbers Oxidation number • A number assigned to an element in a compound that indicates the general distribution of electrons in the compound. Rules for Assigning Oxidation Numbers 1. Atoms in a pure element (Na or O2) have an oxidation number equal to zero 2. In a binary compound the more electronegative element gets a negative charge and the other gets a positive charge. 3. Fluorine always has an oxidation # = -1 4. Oxygen usually has an oxidation # = -2 Peroxides are an exception (H2O2) where oxidation # = -1 5. Hydrogen has an oxidation # = +1 when bonded to more electronegative elements or -1 when bonded to metals 6. The sum of oxidation numbers is 0 for a neutral compound 7. The sum of oxidation numbers for a polyatomic ion is equal to the charge of the ion Sample Problems a. KClO4 b. SO32- c. NF3 d. CO2 e. NO3f. NH4Cl