Gujranwala Guru Nanak Public School Assignment
advertisement

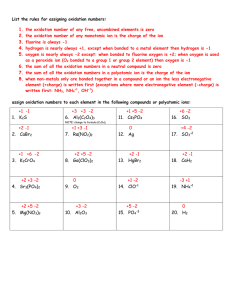
Gujranwala Guru Nanak Public School Assignment- Chemistry Class- XII 1 Account for the following a) Cobalt(2)is stable in aqueous solution but in the presence of complexing reagents it is easily oxidized b) Transition metals and their many compounds act as a good catalyst c) Actinoid contraction is greater from element to element than lanthanoid contraction. Why? 2 Assign a reason a) Sc3+ ion is colourless b) What is the last element in the series of actinoids? Comment on the possible oxidation state of this element 3 Write the steps involved in the preparation of a) K2 Cr2 O7 from Na2 Cr O4 b) Calculate the magnetic moment of a divalent ion in aqueous solution if its atomic number is 25. 4 a) What is meant by lanthanoid contraction? b) Give structures of MnO4- and Cr2O72- ions. 5 a) What is similarity between oxides of lanthanoids and alkaline earth metal oxides b)Why is the highest oxidation state of a metal exhibited in in its oxide or fluoride only? c) What do you mean by interstitial compounds? 6 Why is Cr2+ reducing and Mn3+ oxidizing when both have d4 configuration. 7 Calculate spin only magnetic moment of M2+ (aq) ion. (Z=27). 8 a) Separation of lanthanoids is difficult. Why? b) Why transition elements form many complex compounds? 9 What happens when KMnO4 is heated at 513K? Write its two physical properties also. 10 Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why? 11 Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element. 12 a) Write formula of Potassium tetrahydroxo zincate (II) b) Write IUPAC name of [Cr(NH3)3(H2O)3]Cl3. c) What is ionization isomerism? 13 a) Draw structure of Mn2(CO)10. b) NiCl4 is paramagnetic while Ni(CO)4 is diamagnetic though both are tetrahedral. why? 14 Describe the shape, nature of hybrid orbital & magnetic behavior of the following coordinate entities. i) [ Cr(NH3)6] 3+ ii) Ni(CO)4 (Cr=24, Ni=28) 15 Draw cis and trans structures of [ Pt Cl2 (en)2]+2 .Out of these structure which will show optical activity