Jeopardy - mvhs
advertisement

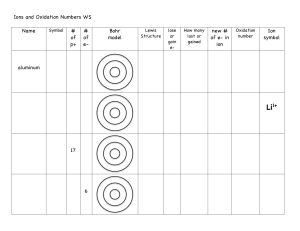
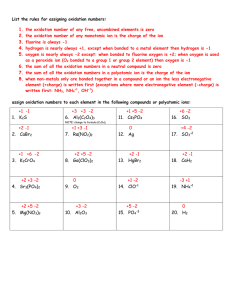
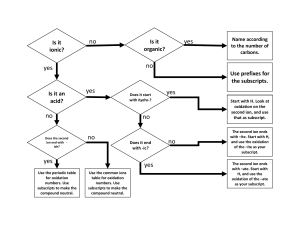
Hosted by YOUR GOOD OLD GROUP 6 Empirical/M olar masses Ions Polyatomic ions 100 100 100 100 200 200 200 200 300 300 300 300 400 400 400 400 500 500 500 500 Oxidation What is an oxyanion? A general term for a polyatomic ion that contains oxygen Row 1, Col 1 name the compound K2C2O4 ? Potassium Oxalate 1,2 What is 62? The molar mass of a nitrate ion 1,3 What is –1? The oxidation of a hydride ion 1,4 What is +2? It is the Charge of a Cobaltous ion 2,1 What is the formula a dichromate ion Cr2O7 2,2 What is about 5%? The percent composition of Hydrogen in an acetate ion 2,3 What is C, N, P, S, Cl, Br, I ? two elements with three or more oxidation states 2,4 What is TWO? The number of atoms in a molecule of a hypochlorite ion 3,1 What is the formula for carbide? C2 2- 3,2 What is 18? The molar mass of a molecule of water 3,3 What is +2? Oxygen’s oxidatin number when in a binary halide 3,4 What is the numerical prefix indicating seven atoms of an element in a molecule? “HEPTA-” 4,1 What is the formula for bicarbonate? HCO3- 4,2 What is Avogadro’s Number? 6.022 X 1023 4,3 What is 0? The oxidation number of a pure element 4,4 What is +4 It is the charge of a stannic ion 5,1 What is Carbide, Imide, or Polysulfide? a A non-oxyanion with a charge of 2- 5,2 What is N3H6? The empirical formula is NH2 and the molecular formula’s molar mass is about 48 grams 5,3 What is -1? Oxidation state of Fluorine 5,4