Oxidation Number lesson plan final
advertisement

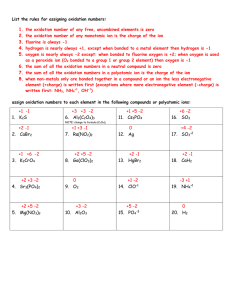
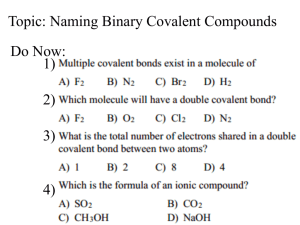
Oxidation Number Lesson Plan Emily Qu =D Duration Activity Plan 5 min Review 1. What do you remember about atomic numbers? (# of positive charge) 2. If we know the number of proton an atom has, what can we predict about the number of electrons? Why are the equal? (Achieve Stability) 3. Every atom of every element has e- arranged in energy levels. Energy levels corresponds to the period or row that the element is located (ex. Na in alkali metal, 3rd row-> electrons are located in 3 energy levels) 4. Draw energy level diagram for sodium. Atomic number is 11-> 11 proton and electron -draw 3 circles around Na nucleus -on inner circle, max of 2 e-on the next circle, max of 8 e-So far we have 10 e-, we have 11 in total -for a chemical reaction to occur, energy level must be completely full or empty, must give up 1e- or gain 7e- for a successful reaction -compare # of proton and electron, uneven, this is the oxidation number 5. If class is confused, go over ON for Li. Discuss similarities between Na and Li 15 min Lesson Hand Out Work Sheet (Attached) 1. The Elements: The oxidation number of a pure element is zero e.g. Fluorine in F2 has ON of 0 2. Simple Ion Charge = Oxidation Number e.g. Na1+ has ON +1, Mg2+ has ON +2 3. Hydrogen: Hydrogen has ON +1 in all compounds except in metal hydrides where it is -1. e.g. Water: H is bonded to O by a polar covalent bond. The e- pair is closer to O -> H is partially positive (δ+) and O is partially negative (δ-). H has ON of -1. 4. Oxygen O usually has ON of -2, except in peroxides where it is assigned -1, and in OF2 where it is assigned a +2 due to the higher electronegativity of F. -In calcium oxide, CaO, oxide ion has a 2- charge. Its oxidation number is -2 -In H2O2, Each O is assigned the ON of -1 15min Group Divide the class up into group of 4. Each group has to come up with 8 Activity different ions. Each ion has to correspond to a different ON ranging from 3 to +3 with no repeats. Students then have to pair up their elements to 15min Lesson Cont. form compounds.(for example, Na+ is +1, Cl- is -1, they can be paired together. Mg2+ is +2, O2- is -2, and they can be paired together). Take up answer in class. 5. Covalent Compounds: In compounds that do not contain hydrogen or oxygen, the more electronegative element is assigned the oxidation number it would have in an ionic compound. (Provide electronegativity table) e.g. In SCl2, chlorine has a higher electronegativity so it is assigned -1 first and sulfur is assigned +2. 6. Compounds: The sum of the ON in a compound is zero. e.g. The sum of ON in NaCl is (+1) + (-1) = 0 7. Polyatomic Ions The sum of the oxidation numbers of the elements in a polyatomic ion must equal the ion charge. Consider these examples. e.g. Carbonate ion, . O has ON of -2. There are 3O in the formula so the total negative charge is 6-. Since the carbonate ion has a charge of 2-, the ON of C must be +4. 20min (if time allows) Game Bingo game: Give each student a different bingo sheet. They will have to calculate ON of certain ions or molecules in a compound from the questions given (try to pick your questions out of a hat so it looks fair). Students then circle the corresponding ON on their bingo sheet. The first student to get a row, a column, or a diagonal line wins Bingo sheet example: +1 +5 -2 +2 +7 -1 0 +6 0 +2 -1 -2 +4 +1 -3 +3 Questions: - elements not combined with others? (0) - oxygen in compounds (-2) - A metal which has lost 4 electrons (+4) - Iron in the compound Iron (III) oxide (+3) - Cl in Cl- (-1) - Mn n MnO4-(+7) - S in SO42- (+6) - N in NO3- (+5) - Nitrogen in ammonia (-3) - Chlorine in ClO3- (+5) - Cu in Cu2+ (+2) - Na in Na+ (+1) Take up answers Note: ON=oxidation number e- = electron