Document 11123970
advertisement
Name:_ _.:...~.:...e_'1,--______
13 Nov 2015
Prof. Urban
5C225 Exam, Chapters 7, 8
Your job is to convince me you know what you are doing. Use your time wisely. Explanations needn't be
long!
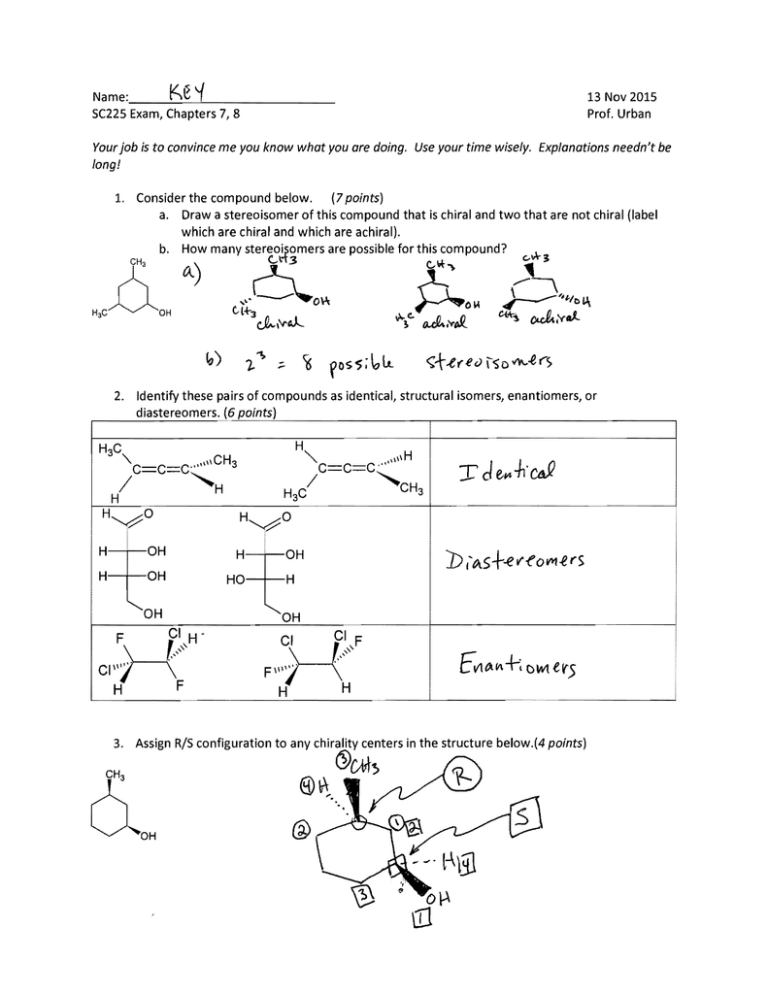
1. Consider the compound below.
(7 points)
a. Draw a stereoisomer of this compound that is chiral and two that are not chiral (label
which are chiral and which are achiral).
b. How many stereoisomers are possible for this compound?
Ik
D
CHa
HaC
2.
)~O"
0..
OH
c.,*~'
(.,\-t3
)
C-t+3 _ n
oA,<!­
_I
~~~
~
/i.O"
_
n
n
~~~
3
P'''Iol\
C(.~
-
{J
~J...
~I
Identify these pairs of compounds as identical, structural isomers, enantiomers, or
diastereomers. (6 points)
H
H3C
"C==C==C<CH 3
/
H
H~O
.-:?'
H
H3C/
H=t
HO
OH
)-_f'H­
"
",
H
'"
C==C==C'"
F
.,111
H
r
del4.f,·W
CH 3
H~O
.-:?'
:t~:
CI III'"
'"
oH
D('t\S +-e V' ofO;'l1-€ r S
H
OH
}_f'F
FIlII'"
'
fv1(A(t\ ~ OINt e"'5
""
H
H
i
~
3.
~
Assign R/5 configuration to any chirality centers in the structure below.(4 points)
CiOH
®~
6)Vbt~
'..
~
4. Structure 1 below is one of the stereoisomers possible for l,4-dibromopentane. {15 points}
yy
Br
Br
1
a. Provide structures of the remaining possible stereoisomers and label each with an
identifying number (2, 3, etc.) and use these numbers when answering the remaining
questions.
b. Are any ofthe structures duplicates (Le., identical compounds)? If so, which?
'1..eS
c. Which stereoisomers are chiral?
\
~
2.<t
.
fJ
3 cure...
\
tso
{
q ()/{~ \deK+ic.a..l1... fA--
I/f\
.
n)
e6""pO~
.0
CJfA.' r~
d. Which of the structures are enantiomers of #1?
3 \s +h.e
e. Which ofthe structures are diastereomers of #1?
2/£.f
\5
fA....
1-
.evtllVli
d\" ll.S +.e
r'.(
{O
~ 1­
~ e-r
o""'""~
1-
1-.
S. True or False. If a statement is false, state why it is false. (9 points)
a. If a single enantiomer of a chiral compound is subjected to archiral reaction conditions,
L. AVO
the product formed must also be a single enantiomer of a chiral compound. j
<"";) _
I.
• ... IJ.J.
S
re~Ti I I " .
~C./IA')4-"KtM. ~?~ M- I~,- ~
Z,.,.
ISn..)- :j.t..e.
}- ~
Q../-.{)::..
foss . h;li~. (lO>1s/J&-)<
~vt2~,.J'
I
(061, fl- 9;>"/..
M17f:
(JS.e.
A- fd../!..I."",,'c /1"1''1 Vf
(M.{""
tf
'?w 2.I
.f7v
J-::t
t' f1 5fa:.r. (.1...
fL
tv'"
b. According to t e ammon ostulate, endergonic reaction steps have early transition
states.
fJ~ f'iY\dl.K~l
.s4<fS"
-t.,~
J:;t<
rF\7
~)I·-h-h\.
Sf.,~.
(l.lC 1\ CoffrJ..
c. The rate of an SN1 is expected to depend on leaving group ability.
~
~ ~ ~ ~ let\,"(~
-g 1"
ad rJL ~ ~-cJe}ey~·n/"j,.
r-~ (/2--' ff
{
U
s+r.
6. The rate law for the reaction below has been found by experiment to be Rate = k[CH3hCBr].
(15 points)
a. Provide a mechanism for the reaction.
b. Provide a plot of Energy vs Reaction Coordinate. Label the plot to indicate the positions
of key structures such as transition states and intermediates.
c. Do you expect the rate of reaction to increase or decrease if the solvent polarity is .
:S ~~
increased? Explain.
~)
---:;:- 1rM'~1 \6'1\
-+.
H C
3 \
C/
H C/ \
3
CH ..
3
CH 30H
Br
H3C
\
3
.....
C..... CH
H C/ \
3
r
:t
IG
OCH 3
fl.""", Cotx-tJ.
7. Provide a mechanism for the transformation shown below. (8 points)
H'X~.~H4r
V:;H
b
Wf
\.t
~
HBr·
H,G>( _
UGH,
~~l
t~
(}/If @~1-1 1tD"j4 ~
Il<S'cAt,
)~
LJ
-?
(Ij_<tl tIl")
{
"".. .A",( r-iM
!i..,Y'"
Jil'if~
8. Which ofthe following will give rise to the most stable carbocation and why? Write a number
under each ranking them from 1 to 3 with 1 most stable carbocation and 3=least stable
carbocation. (3 points)
Br l ~ 2..- ti.I\;t
Br
fl.$M(),N<-U-
s--htc,,) /z4.
i~~~>O
1-
bh
w.(Jf~
ey.+ud(cl
~
10. For each pair, circle the better nucleophile and state your reasoning? (4 points)
@
a. OH
or
H20
11 "Sr~
S+r~ .t,(v.)L -;; lo.e,~ V\.~d.t.()ol.,; Lc. CW'
.\D~ltv I 'rv'" '"
0
L"
,.,'aL
- ~
._.
~G\. V""'-
'('
0 "V
OJ..... ·od..L
"""....'
I
11. For each pair of reactions, state which reaction is expected to exhibit a faster SN2 rate and why.
(8 points)
a.~
+ NaI
acetone
.
1 svIP s-/yo. +e. r ltJ..cks ~s~ I
C)
Sr
a.
~
01s
,"" 5tJ 2- ~ ;). 0 •
+ NaOH
acetone
.
fJ [.\'J"-
\s
~~ ()l~
+ NaNH2
is
~{
,o,tJ
fA.
­
~ /J,,­
b.ee-~ r~
Io~~(...
1"'-
row
() ~ p,w,
~
.~ -f,~G-)
12. Fill in the blanks. Provide the missing reactant{s), product{s), or reagent{s). (12 points)
Br
._ ~~3
1>(.,\,1'4
~ CH,o~
?
0 ' .. ~
~
0\')
0~
TsCI
Pyridine
rAC.e-''­
i
NaBr
DMSO
..
?
GJ
?
c.rJ -
DMSO
+ Nal
-
acetone
?
13. For each reaction below, state if the reaction will proceed primarily by an SNl mechanism or
primarily ban SN2 mechanism. Give your reasoning.
(6 points) Note, the question isn't asking you to provide the products.
s.!VO{'1 SI'
TSO~
+ NaOCH 3
{MClA (iJYI)ff< ,;:~~/;;
r CMt,6~fJ'()'\)