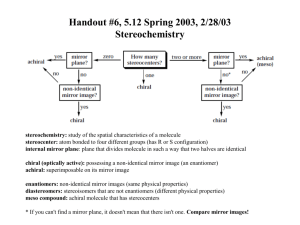
5-4 Meso Compounds PPT
advertisement
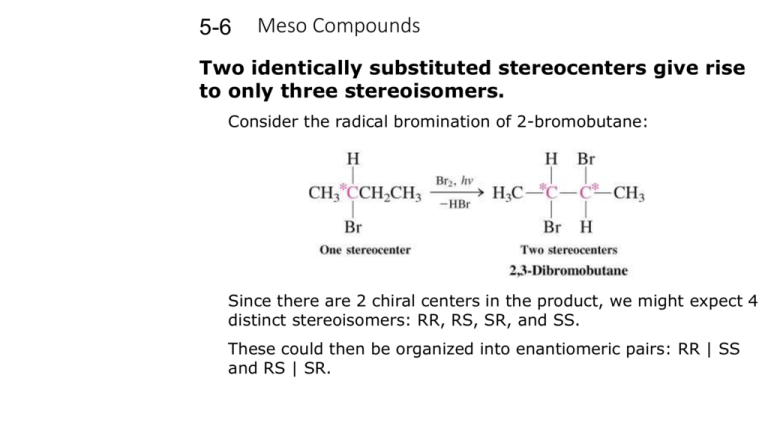
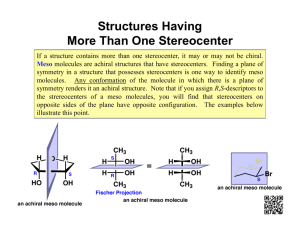
5-6 Meso Compounds Two identically substituted stereocenters give rise to only three stereoisomers. Consider the radical bromination of 2-bromobutane: Since there are 2 chiral centers in the product, we might expect 4 distinct stereoisomers: RR, RS, SR, and SS. These could then be organized into enantiomeric pairs: RR | SS and RS | SR. A closer look at the RS and SR pair of molecules, however, shows that they are superimposable molecules and are therefore identical. A compound containing 2 or more stereocenters that is superimposable with its mirror image is called a meso compound. Meso compounds contain an internal mirror plane which divides the two halves of the molecule which are mirror images of each other. The presence of a mirror plane in any energetically accessible conformation of a molecule is sufficient to make it achiral. 2,3-Dibromobutane exists as three stereoisomers only: a pair of enantiomers and an achiral meso diastereomer. Examples of meso compounds having multiple chiral centers: