Behavior of gase -power point
advertisement

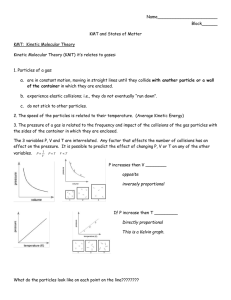
The Behavior of Gases Three measurable properties of gases: 1. Volume: The amount of space a gas occupies depends on temperature and air pressure. Gases fill all available space so the volume of a gas depends on the size of the container. 2. Temperature: the average energy of motion of the particles in a substance. The faster the particles move, the greater the temperature. (Example: At room temperature particles in a gas move at 500 m/s.) 3. Pressure: the force of the push from the particles of a substance on the walls of the container in which the gas is contained. (Remember: P = F/area) Gas particles move from areas of high pressure to areas of low pressure until equilibrium is reached. Relationships between the properties of gases: 1. The relationship between pressure and volume is known as Boyle’s Law. Boyle’s Law states that, at a constant temperature, when the pressure of a gas increases the volume decreases. When the pressure decreases the volume increases. This relationship is inversely proportional. Example: Compressed gases exert greater pressure such as a CO2 cartridge. 2. As the temperature of a gas at a constant volume increases so does the pressure. Heat provides energy to the particles in the gas causing them to move more rapidly increasing the collisions. The more collisions that occur the greater the pressure. As the temperature decreases energy also decreases thus decreasing the number of collisions of gas particles. The decreased number of collisions results in decreased pressure. This relationship is directly proportional. Example: Tire pressure increases in warm weather and decreases in cold weather. 3. The relationship between volume and temperature is known as Charles Law. Charles Law states that at a constant pressure, when the temperature of a gas increases its volume also increases. When the temperature of a gas decreases, its volume decreases. This relationship is directly proportional. As the temperature increases, the gas particles increase motion. The volume of a gas must increase to keep rate of collisions constant thus, keeping the pressure constant. If the volume of a container of gas can change, the total force from the collisions results in the gas taking up more space. Example: Hot air balloon – as air is heated it fills up the balloon. Law Volume Temperature Pressure Relationship Boyle’s Increase Constant Decrease Inverse Boyle’s Decrease Constant Increase Inverse Charles Increase Increase Constant Directly Charles Decrease Decrease Constant Directly Pressure and Temperature Constant Increase Increase Directly Pressure & Temperature Constant Decrease Decrease Directly