3.2 pp
advertisement
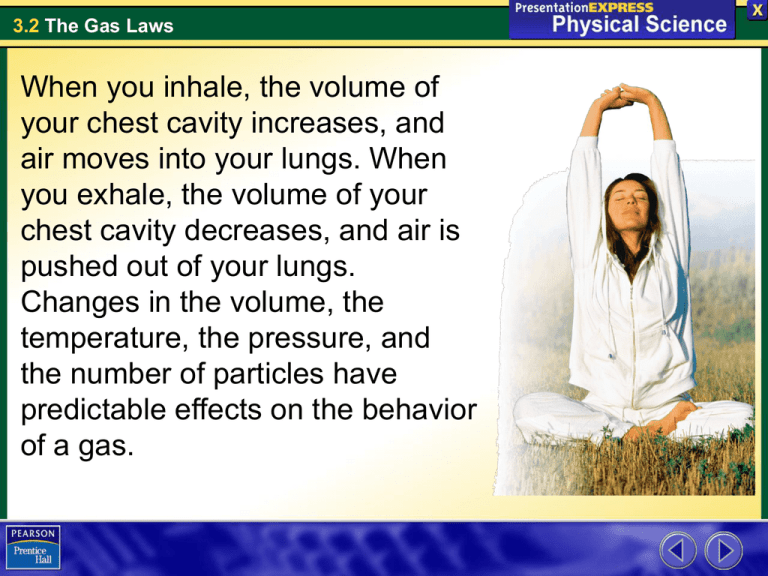
3.2 The Gas Laws When you inhale, the volume of your chest cavity increases, and air moves into your lungs. When you exhale, the volume of your chest cavity decreases, and air is pushed out of your lungs. Changes in the volume, the temperature, the pressure, and the number of particles have predictable effects on the behavior of a gas. 3.2 The Gas Laws Pressure What causes gas pressure in a closed container? Pressure is the result of a force distributed over an area. Collisions between particles of a gas and the walls of the container cause the pressure in a closed container of gas. 3.2 The Gas Laws Pressure A moving hockey puck exerts pressure on any object it hits. A layer of shatterproof glass protects spectators. • The faster the puck is traveling, the greater the force of the puck on the glass. A greater force means more pressure. • The smaller the area of impact is, the greater the pressure. If the edge of the puck hits the glass, it exerts more pressure than if the face of the puck hits the glass. 3.2 The Gas Laws Pressure The SI unit of pressure is derived from SI units for force and area. • Force is measured in newtons (N). • Area is measured in square meters (m2). • The SI unit for pressure, the pascal (Pa), is shorthand for newtons per square meter. • Scientists often express larger amounts of pressure in kilopascals. One kilopascal (kPa) is equal to 1000 pascals. 3.2 The Gas Laws Pressure The helium atoms in a balloon are constantly moving. There are more than 1022 helium atoms in a small balloon. • When many particles collide with the walls of a container at the same time, they produce a measurable pressure. • The more frequent the collisions, the greater the pressure is. • The speed of the particles and their mass also affect the pressure. 3.2 The Gas Laws Factors That Affect Gas Pressure What factors affect gas pressure? Factors that affect the pressure of an enclosed gas are its temperature, its volume, and the number of its particles. 3.2 The Gas Laws Factors That Affect Gas Pressure Temperature Raising the temperature of a gas will increase its pressure if the volume of the gas and the number of particles are constant. 3.2 The Gas Laws Factors That Affect Gas Pressure The firefighter is using a pressure gauge to check the air pressure in a tire on a firetruck. If he checks the tire pressure again after a long drive on a highway, he will find that the pressure has increased. 3.2 The Gas Laws Factors That Affect Gas Pressure The motion of tires on the highway heats the tires and increases tire pressure. • As the temperature rises, the average kinetic energy of the particles in the air increases. • With increased kinetic energy, the particles move faster and collide more often with the inner walls of the tires. • Faster-moving particles hit the walls with greater force. • More collisions and increased force cause the pressure of the air in the tires to rise. 3.2 The Gas Laws Factors That Affect Gas Pressure Volume Reducing the volume of a gas increases its pressure if the temperature of the gas and the number of particles are constant. 3.2 The Gas Laws Factors That Affect Gas Pressure Twist the cap onto a plastic bottle and then squeeze it. What happens? • The volume of the plastic bottle begins to decrease. • As the volume decreases, the particles of trapped air collide more often with the walls of the bottle. • The pressure in the bottle increases. 3.2 The Gas Laws Factors That Affect Gas Pressure Movement of a muscle called the diaphragm changes the volume of your chest cavity. • The volume increases when you inhale. The pressure decreases and air flows to your lungs. • The volume decreases when you exhale. The pressure increases and air flows from your lungs. Inhaling Diaphragm contracts. Rib cage is lifted up and out. Exhaling Lungs Rib Cage Diaphragm Diaphragm relaxes. Rib cage moves down and in. 3.2 The Gas Laws Factors That Affect Gas Pressure Number of Particles Increasing the number of particles will increase the pressure of a gas if the temperature and the volume are constant. The more particles there are in the same volume, the greater the number of collisions and the greater the pressure. 3.2 The Gas Laws Charles’s Law French physicist Jacques Charles collected data on the relationship between the temperature and volume of gases. The graph of the data showed a direct relationship between the volume of a gas and the temperature of the gas. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law Charles extended the graph beyond the measured data to find the temperature that would produce a volume of 0 L. •The temperature at the point where the line crossed the x-axis was –273.15°C. 3.2 The Gas Laws Charles’s Law •This temperature is equal to 0 K on the Kelvin temperature scale. •A temperature of 0 K is called absolute zero. 3.2 The Gas Laws Charles’s Law Charles’s law states that the volume of a gas is directly proportional to its temperature in kelvins if the pressure and the number of particles of the gas are constant. T1 and V1 represent the temperature and volume of a gas before a change occurs. T2 and V2 represent the temperature and volume after a change occurs. 3.2 The Gas Laws Boyle’s Law Robert Boyle described the relationship between the pressure and volume of a gas. The graph shows an inverse relationship between the volume of a gas and the pressure of the gas. 3.2 The Gas Laws Boyle’s Law Robert Boyle described the relationship between the pressure and volume of a gas. The graph shows an inverse relationship between the volume of a gas and the pressure of the gas. 3.2 The Gas Laws Boyle’s Law Robert Boyle described the relationship between the pressure and volume of a gas. The graph shows an inverse relationship between the volume of a gas and the pressure of the gas. 3.2 The Gas Laws Boyle’s Law Robert Boyle described the relationship between the pressure and volume of a gas. The graph shows an inverse relationship between the volume of a gas and the pressure of the gas. 3.2 The Gas Laws Boyle’s Law Boyle’s law states that the volume of a gas is inversely proportional to its pressure if the temperature and the number of particles are constant. P1 and V1 represent the pressure and volume of a gas before a change occurs. P2 and V2 represent the pressure and volume of a gas after a change occurs. 3.2 The Gas Laws The Combined Gas Law The relationships described by Boyle’s law and Charles’s law can be described by a single law. The combined gas law describes the relationship among the temperature, volume, and pressure of a gas when the number of particles is constant. 3.2 The Gas Laws The Combined Gas Law The Combined Gas Law A cylinder that contains air at a pressure of 100 kPa has a volume of 0.75 L. The pressure is increased to 300 kPa. The temperature does not change. Find the new volume of air. 3.2 The Gas Laws The Combined Gas Law Read and Understand What information are you given? 3.2 The Gas Laws The Combined Gas Law Read and Understand What information are you given? P1 = 100 kPa P2 = 300 kPa V1 = 0.75 L 3.2 The Gas Laws The Combined Gas Law Plan and Solve What unknown are you trying to calculate? What expression can you use? 3.2 The Gas Laws The Combined Gas Law Plan and Solve What unknown are you trying to calculate? What expression can you use? 3.2 The Gas Laws The Combined Gas Law Plan and Solve Cancel out the variable that does not change and rearrange the expression to solve for V2. Replace each variable with its known value. 3.2 The Gas Laws The Combined Gas Law Plan and Solve Cancel out the variable that does not change and rearrange the expression to solve for V2. Replace each variable with its known value. 3.2 The Gas Laws The Combined Gas Law Look Back and Check Is your answer reasonable? 3.2 The Gas Laws The Combined Gas Law Look Back and Check Is your answer reasonable? Volume should decrease as pressure increases. The pressure tripled from 100 kPa to 300 kPa. The answer, 0.25 L, is one third the original volume, 0.75 L. 3.2 The Gas Laws The Combined Gas Law 1. A gas has a volume of 5.0 L at a pressure of 50 kPa. What happens to the volume when the pressure is increased to 125 kPa? The temperature does not change. 3.2 The Gas Laws The Combined Gas Law 2. Gas stored in a tank at 273 K has a pressure of 388 kPa. The safe limit for the pressure is 825 kPa. At what temperature will the gas reach this pressure? 3.2 The Gas Laws The Combined Gas Law 3. At 10ºC, the gas in a cylinder has a volume of 0.250 L. The gas is allowed to expand to 0.285 L. What must the final temperature be for the pressure to remain constant? (Hint: Convert from degrees Celsius to kelvins using the expression ºC + 273 = K.) 3.2 The Gas Laws The Combined Gas Law Balloons like this one are used by scientists to gather data about Earth’s atmosphere. The balloon is filled with hydrogen or helium. It carries a package of weather instruments up into the atmosphere. 3.2 The Gas Laws The Combined Gas Law The gas laws explain the behavior of the gas in the balloon. 3.2 The Gas Laws Assessment Questions 1. What causes the pressure to increase if more gas particles are added to a closed container? a. an increase in the number of collisions between the gas and the container walls b. a decrease in the volume of the container c. a decrease in the size of each particle as the number of particles increases d. an increase in the number of collisions between air particles and the outside of the container 3.2 The Gas Laws Assessment Questions 1. What causes the pressure to increase if more gas particles are added to a closed container? a. an increase in the number of collisions between the gas and the container walls b. a decrease in the volume of the container c. a decrease in the size of each particle as the number of particles increases d. an increase in the number of collisions between air particles and the outside of the container ANS: A 3.2 The Gas Laws Assessment Questions 2. When first blown up, a balloon is firm because of the air pressure inside it. However, after time, the balloon becomes soft as the air pressure inside drops. What could have caused the air pressure to decrease? a. increase in air temperature b. decrease in the balloon's volume c. decrease in the number of air particles as they leaked out of the balloon d. a chemical reaction between the air particles and the balloon 3.2 The Gas Laws Assessment Questions 2. When first blown up, a balloon is firm because of the air pressure inside it. However, after time, the balloon becomes soft as the air pressure inside drops. What could have caused the air pressure to decrease? a. increase in air temperature b. decrease in the balloon's volume c. decrease in the number of air particles as they leaked out of the balloon d. a chemical reaction between the air particles and the balloon ANS: C 3.2 The Gas Laws Assessment Questions 3. A gas has a volume of 15 L, a temperature of 300 K, and an unknown initial pressure. Then, the gas expands to 30 L, remains at 300 K, and has a pressure of 300 kPa. What was the initial pressure of the gas? a. b. c. d. 150 kPa 600 kPa 330 kPa 570 kPa 3.2 The Gas Laws Assessment Questions 3. A gas has a volume of 15 L, a temperature of 300 K, and an unknown initial pressure. Then, the gas expands to 30 L, remains at 300 K, and has a pressure of 300 kPa. What was the initial pressure of the gas? a. b. c. d. 150 kPa 600 kPa 330 kPa 570 kPa ANS: B 3.2 The Gas Laws Assessment Questions 4. According to Charles’s law, the relationship between the temperature and the volume of a gas is a. b. c. d. direct. inverse. exponential. inverse square. 3.2 The Gas Laws Assessment Questions 4. According to Charles’s law, the relationship between the temperature and the volume of a gas is a. b. c. d. direct. inverse. exponential. inverse square. ANS: A 3.2 The Gas Laws Assessment Questions 1. When the temperature of the gas in closed container is increased, the pressure increases. True False 3.2 The Gas Laws Assessment Questions 1. When the temperature of the gas in closed container is increased, the pressure increases. True False ANS: T