Name_______________________ Block______ KMT and States of
advertisement
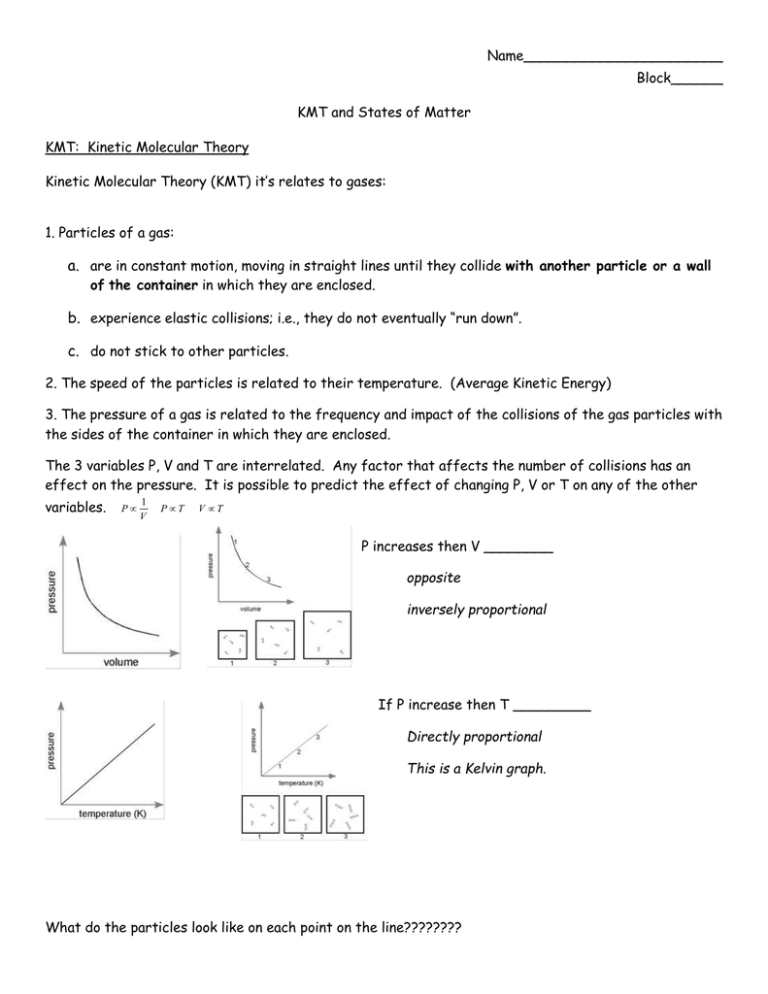
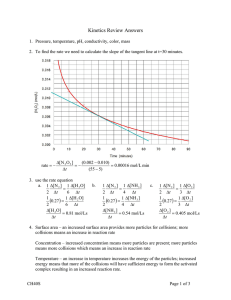
Name_______________________ Block______ KMT and States of Matter KMT: Kinetic Molecular Theory Kinetic Molecular Theory (KMT) it’s relates to gases: 1. Particles of a gas: a. are in constant motion, moving in straight lines until they collide with another particle or a wall of the container in which they are enclosed. b. experience elastic collisions; i.e., they do not eventually “run down”. c. do not stick to other particles. 2. The speed of the particles is related to their temperature. (Average Kinetic Energy) 3. The pressure of a gas is related to the frequency and impact of the collisions of the gas particles with the sides of the container in which they are enclosed. The 3 variables P, V and T are interrelated. Any factor that affects the number of collisions has an effect on the pressure. It is possible to predict the effect of changing P, V or T on any of the other variables. P 1 V P T V T P increases then V ________ opposite inversely proportional If P increase then T _________ Directly proportional This is a Kelvin graph. What do the particles look like on each point on the line???????? Comparing the number of particles (n) to pressure…………. Remembering the Can Activity, complete the following: When the can was heated, the water turned to ___________, which takes up ________ (more/less) volume than liquid water. When the can was inverted into the water this created a closed system. The temperature inside the can ___________ (increased/decreased), causing the steam to change from gas state to ______________state. A partial vacuum was created causing the pressure inside the can to be ______________ (greater/less) than the pressure outside the can. Complete the following: If the volume of a gas is cut by 1/2, the pressure will _____________________ (increase/decrease) by a factor of _________ (2, 1/2) times. If the temperature of a gas is doubled, the volume of the gas will ____________ (increase/decrease) by a factor of ___________ (2, 1/2) times. If the temperature of a gas is tripled, the pressure of the gas will ____________ (increase/decrease) by a factor of _________ (3, 1/3) times. Graphing pressure vs. volume would produce a Graphing volume vs. temperature would produce a _____________ line. _______________.