The Gas Laws
advertisement

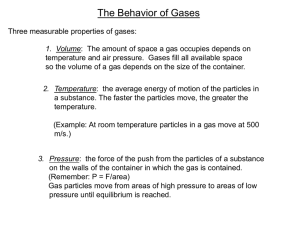
The Gas Laws What are the Gas Laws? The gas laws deal specifically with volume, temperature, and pressure. Changes in volume, temperature, and pressure have predictable effects on the behavior of gases Volume, Temp., Pressure, and the # of particles in a container of gas all affect one another Pressure Pressure is the result of a force distributed over an area Pressure (atm) is measured in atmospheres Collisions between particles of a gas and the walls of the container cause the pressure in a closed container of gas Pressure in Airplanes Before an airplane takes off, the plane must regulate its air pressure inside the cabin As the airplane takes off higher into the sky, the pressure on the outside of the plane is too dangerous, so the cabin must keep a safe, regulated air pressure sealed inside A difference in pressure is also why your ears “pop” Factors that affect gas pressure Factors that affect the pressure of an enclosed gas are its temperature, its volume, and the number of particles Remember: in a gas the particles are constantly moving and bumping into one another Temperature The constant motion of particles in a gas cause the gas to heat up, this is due the gas’ kinetic energy. As temperature rises, the average kinetic energy of the gas particles also increases Temperature cont. The increase in the number particle collisions, along with the increase in the force of the collision causes an increase in pressure within the container Raising the temperature of a gas will increase its pressure if the volume of the gas and the number of particles are constant (Stay the same) Volume As the volume in a container of gas is decreased, particles of trapped air start to collide more often with one another and the container’s walls Volume cont. Reducing the volume of a gas increases its pressure, as long as the temperature of the gas and the amount of gas particles remains constant (stays the same) Number of Gas Particles When you add more gas particles to a container of gas, the pressure will start to increase Think about adding more air to a balloon that is already full, the pressure increases and eventually the balloon will pop Number of Gas Particles Cont. Increasing the number of particles in a container of gas will increase the pressure of the gas, as long as the temperature and volume remain constant (stays the same) Charles Law Named after French, physical scientist Jaques Charles. His He inventions include the helium balloon is also known for his study on how gases behave Charles Law cont. Charles law states: “The volume of a gas is directly proportional to its temperature (in kelvins, k) if the pressure and the number of particles of a gas are constant.” Charles Law cont. To convert Celsius to Kelvin, simply add 273 to the temperature in Celsius Example: 30°C = 273+30 = 303°K Charles Law cont. The this: equation for Charles Law looks like Boyle’s Law Robert He Boyle was an Irish scientist was the first to describe the relationship between the pressure and volume of a gas Boyle’s Law cont. Boyle’s Law states: “The volume of a gas is inversely proportional to its pressure if the temperature and number of particles in a gas remain constant.” Boyle’s Law Boyles Law is expressed like this: The Combined Gas Law The relationships described by Boyle’s Law and Charles’ Law can be described by a single law As long as we know the number of particles remains constant, we can use the combined gas law The Combined Gas Law cont. The Combined Gas Law looks like this: Combined Gas Law cont. The Ideal Gas Law Now, all this math and chemistry is probably making your brain want to explode Good news, there is a simpler gas law! The Ideal Gas Law The Ideal Gas Law looks like this PV = nRT R = 0.0821 P = Pressure V = Volume T = Temperature n = number of moles in a substance What is R? R will always be 0.082, it’s a constant Crash Course, IGL Crash Course