Behavior of Gases
advertisement

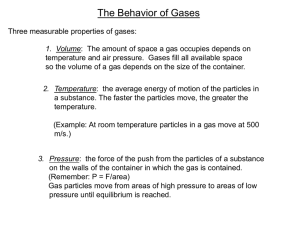
Behavior of Gases Chapter 2 Section 2 Measuring Gases When you think of a gas, what kinds of gases do you think of? Helium, oxygen, carbon dioxide What can affect the volume of a gas such as helium? Temperature Air pressure Volume Volume is the amount of space that matter fills. Volume is measured in cubic centimeters, milliliters, liters, and other units. Because a gas fills the space available, the volume of a gas is the same as the volume of its container. Temperature Temperature is the measure of the average energy of motion of the particles of a substance. The faster the particles are moving, the greater their energy and the higher the temperature. You might think of a thermometer as a speedometer for molecules. On an ordinary day, the particles in a gas move very fast. At room temperature, the particles in a typical gas travel about 500 meters per second. (1500 feet per second) Pressure Because gas particles are moving, they constantly collide with the walls of their container. As a result, the gas exerts an outward push on the walls of the container. The pressure of a gas is the force of its outward push divided by the area of the walls of container. Pressure is measured in units of kilopascals (kPa). Pressure = Force Area About Gases The firmness of an object inflated with a gas, such as a soccer ball, comes from the pressure of the gas. If the gas leaks from the ball, the pressure decreases. A gas flows from high pressure to low pressure. The air inside the ball has a higher pressure than the air outside the ball, so the gas flows out. The pressure inside the ball drops until it is equal to the pressure outside of the ball. Relating Pressure and Volume Pressure is also related to the volume of the container. In the 1600’s an English scientist, Robert Boyle was experimenting with ways to improve air pumps. He found that gases behave in predictable ways… Boyle’s Law Boyle found that when the pressure of a gas is increased at constant temperature, the volume of the gas decreases. When the pressure is decreased, the volume increases. The relationship between the pressure and volume of a gas is called Boyle’s Law. Where does Boyle’s Law Apply? Boyle’s Law plays an important role in research done with high-altitude balloons. Balloons made of lightweight plastic were filled with small amounts of helium and released into the atmosphere. Scientists found that as the balloon rises through the atmosphere, the air pressure around it decreases steadily, while the helium inside expands, stretching the balloon to a greater and greater volume. If the balloon were full at takeoff, it would have burst from the change in air pressure before it got very high. Pressure and Temperature Temperature is the measure of the average speed of the particles of a gas. The higher the temperature of a gas, the faster the particles are moving. When the temperature of a gas at constant volume (in a closed, rigid container) is increased, the pressure of the gas increases. When the temperature is decreased, the pressure of the gas decreases. Pressure and Temperature in Action Think about a 18 wheeler. The tires on these trucks must be very large, heavy, and stiff in order to support the weight of the truck. On a long trip, in the summer, a truck’s tires can get very hot. As the temperature increases, so does the pressure inside the tire. If the pressure becomes higher than the tire can hold, the tire will burst apart. Relating Volume and Temperature Gas increases in volume the the temperature increases. When the temperature decreases, the volume with decrease. This is important, especially to the people who are in charge of large balloons in parades. What do the people in charge of the Macy’s Day Parade need to take into consideration so that they make sure the balloons are filled properly? Charles’ Law In the late 1700s, a French scientist, Jacques Charles, examined the relationship between the temperature and volume of a gas kept at a constant pressure. He measured the volume of a gas at various temperatures in a container whose volume could change. He found that when the temperature of a gas increased at constant pressure, its volume increases. When the temperature of a gas decreases, its volume also decreases. Because the particles of a gas move fast at higher temperatures, they collide more often with the walls around them. As long as the volume of the container can change, the total push of the collisions results in the gas taking up more space Charles Law in Action Look on page 55 at Figure 15. In the photograph on the left, a balloon in a beaker of water, resting on a tub of ice, shows how the balloon stays smaller. In the photograph on the right, the balloon in the beaker is sitting on a hotplate. We can see the difference in the balloon where the increase in temperature results in an increase in volume. Drawing a Picture