Gas Laws: Boyle's, Charles', Pressure-Temperature
advertisement

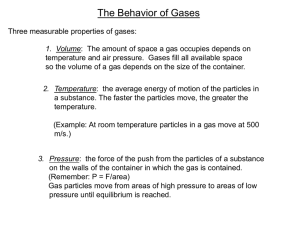
Chapter 16.3 The Gas Laws Pressure and Volume (Boyle’s Law) Temperature and Volume (Charles’ Law) Temperature and Pressure Law Volume, temperature and pressure are three different properties of a gas you can measure. Volume, temperature and pressure are are related. How? The Gas Laws ! The three properties of a gas are: Volume: The amount of space it takes up. The volume of a gas will be the volume of its container.(Liters) Temperature: The temperature of a gas is a measure of the average kinetic energy of the moving particles. If the particles are moving faster, their energy will be greater and the temperature will be higher. Pressure: The pressure of a gas is the force of its outward push divided by the area of the walls of the container. Measured in Pascals (Pa), atmospheres (atm), pounds per square inch (psi) The three properties of gases—volume, temperature and pressure—are all related. Boyle’s Law Robert Boyle (1627-1691) Boyle conducted pioneering experiments in studying the role of air in combustion, respiration, and the transmission of sound. In 1662, Boyle published what is now known as Boyle's law: At constant temperature the volume of a gas is inversely proportional to the pressure. • What does this mean in words? • V P If the volume goes up then the pressure goes down. The particles have to travel further and hit the containers wall less often. • V P If the volume goes down then the pressure goes up. The particles have to travel less far and hit the containers wall more often. The mathematical form of Boyle's Law is: V1P1 = V2 P2 V1 = intial volume P1 = intial pressure V2 = final volume P2 = final pressure P and V Changes P1 V1 P2 V2 • In this diagram you can see that more mass is added to the top of the plunger at each stage. The more mass, the more the gas is compressed. The same number of gas particles are trapped in an increasingly smaller space, which increases the pressure of the gas on the walls of the container. Boyles Law Practice Solve this problem Initial conditions Final conditions P1 = 50 pascals P2 = 200 pascals V1 = 1.5 L = ? V2 ? Charles Law Jacques Charles (1746-1823) The physical principle known as Charles’ Law states that raising the temperature of a gas increases its volume; lowering the temperature of a gas decreases its volume. Charles’ Law: V and T At constant pressure, the volume of a gas is directly related to its absolute (K) temperature T V T V Charles Law Formula V1 T1 = V2 T2 Remember the temp is measured in K °C + 273 = K In both parts of this diagram the gas is at the same pressure, but you can see that the temperature has risen from –65°C to 250°C. The gas particles move faster at higher temperatures and collide more often. The same number of gas particles now take up a larger space. Charles’ Law Practice V = 125 mL V = 250 mL T = 273 K T = ? Observe the V and T of the balloons. How does volume change with temperature? Note to self. Check balloon in freezer. Another Practice Problem A balloon has a volume of 785 mL on a Fall day when the temperature is 21°C. In the winter, the gas cools to 0°C. What is the new volume of the balloon? Gay-Lussac’s Law: Pressure-Temperature Law The pressure exerted by a confined gas is directly related to the temperature (Kelvin) at constant volume. T P T P Very much like Charles Law! (T and V) Pressure Temperature Law P1 T1 = P2 T2 P T Problem A gas has a pressure at 2.0 atm at 18°C. What will be the new pressure if the temperature rises to 62°C? T = 18°C T = 62°C Learning Check Complete with 1) Increases 2) Decreases 3) Does not change A. Pressure _____, when V decreases B. When T decreases, V _____. C. Pressure _____ when V changes from 12.0 L to 24.0 L D. Volume _____when T changes from 15.0 °C to 45.0°C Solving gas law problems • • • • What information do they give me? (2) What are they looking for? (1) Which of the three formulas applies? Plug in numbers and solve. THE END