Properties of Gases Notes
advertisement

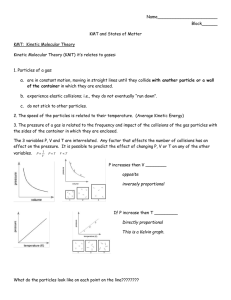
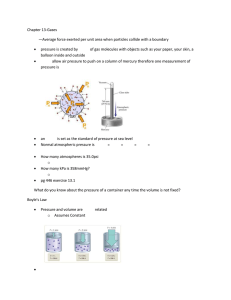
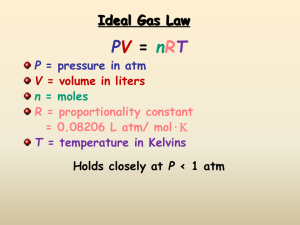
Name:_____________________________________________________________________ Date:_______________________ Period: _____________ Notes on Properties of Gases (Use the “Properties of Gases” PowerPoint) Kinetic Theory and Gases 1. Simple kinetic molecular theory (__________________) can be used to explain the behavior of gases. Sketch what happens when gas molecules collide with the walls of the container. Compressibility of Gases 2. ___________________________________ is a measure of how much the volume of matter can decrease under pressure. 3. According to the KMT gas particles are so __________________ in relation to the distances between them that their individual volumes are virtually insignificant. 4. This means that there is a lot of empty _________________________ between individual gas particles. 5. This space between the particles explains why ________________ can be compressed. Freedom of movement 6. According to the KMT there are no _______________________ or _________________________ forces between gas particles. As a result gas particles are free to move and ________________________ as far as their container will allow them to. Constant random motion 7. The KMT also states that gas particles move in straight _______________________ independently of each other. Only when a particle _____________________________ with another particle or container will their path change. Perfectly elastic collisions 8. The kinetic molecular theory assumes that the collision of gas particles are perfectly elastic and that the total amount of kinetic ___________________________________is conserved within the system. VARIABLES THAT DESCRIBE GASES Pressure (P) Temperature (T) Volume (V) Number of Moles (N) GAS PRESSURE 6. Gas pressure is defined as the force exerted by a gas per unit surface area of an object. This force is the result of simultaneous collisions of billions of gas molecules with the surface of the container. The symbol for pressure is ________ and the unit of measurement is the ___________________________. (STP = 00C at 1 atm) 1 atm = 101.3 Pascal = 760 mm Hg TEMPERATURE 7. According to the KMT the average kinetic energy of a collection of gas is directly proportional to the temperature of the gas when measured in _____________________ units. (0 degrees Celsius = 273 Kelvin) 8. A decrease in temperature makes the volume _________________________. An increase in temperature makes the volume ________________________. VOLUME Volume is the amount of ______________________________ occupied by a substance. The symbol for volume is ______________and it is measured in liters. The volume of any gas at STP = 22.4 L/mol. Number of Moles The number of moles represents the number of _______________________________________ contained in a system. 6.02 X 10 23 particles = 1 mole How can you increase the pressure of a gas? 1. ___________________________________________________________________________________________________________ 2. ___________________________________________________________________________________________________________ 3. ___________________________________________________________________________________________________________ Gas Laws Charles’ Gas Law: Boyle’s Gas Law: Gay-Lussac’s Gas Law: