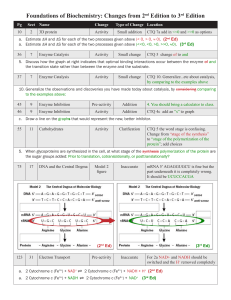
CHAPTER 6 REVIEW SHEET How have mechanisms of enzyme
advertisement

CHAPTER 6 REVIEW SHEET 1. How have mechanisms of enzyme-catalyzed reactions been devised? 2. The extraordinary catalytic ability of enzymes results from what simple properties? 3. What three disciplines have contributed to the understanding of enzyme mechanisms? 4. Identify the mechanism-deriving laboratory technique being described: a. Used to trace the path of individual atoms. b. Measure the changes in the chemical bonds of a reactant or solvent. c. Provide a 3D view of the process. 5. Identify the nucleophile and electrophile, draw the SN1 mechanism and the rate law for the given reaction. O O Ph Ph O Na Cl 6. Bromomethane reacts with sodium hydroxide to produce methanol and sodium bromide. Identify the nulceophile and electrophile, draw the SN2 mechanism and the rate law. 7. Show how the following substances can form using the reactants given: a. carbocation. + C OH2 b. carbanion. NaNH2 C H c. Free radical. R2 h O O R1 8. Determine which species is oxidized and which species is reduced. Identify the oxidizing agent and the reducing agent. 2 K + 2 H2O 2 KOH + H2 a. What type of enzyme catalyzes these types of reactions? b. What is the most common form of biological oxidation, and what catalyzes this process? 9. The rate of a reaction depends on how often the reacting molecules __________ in a way that a reaction is favored. 10. Describe a transition state. 11. For the given energy diagram, label the TS, Ea and ESP. What would it look like if there was an intermediate? Which TS would be the rds? 12. Using the given energy diagram as an uncatalyzed reaction, draw how it would change with (A) the binding of the substrate by an enzyme, and (B) the binding of the substrate and the transitions state by an enzyme. (A) (B) 13. Ionizable side chains in the active site participate in what two modes of chemical catalysis? 14. Fill in the following chart that identifies amino acid residues that have catalytic functions. Amino Acid Reactive Group Functions Aspartate Glutamate Histidine Cysteine Tyrosine Lysine Arginine Serine 15. How can an active site amino acid with an ionizable side chain be identified? 16. Other than participating in the _______________ of catalysis, active site amino acids can… 17. During which type of chemical catalysis must the amino acids be able to donate or accept protons under the nearly neutral conditions of cells? 18. Demonstrate how the base sodium amide can cleave the O–H bond in an alcohol. O H NaNH2 19. Demonstrate how a base like sodium ethoxide can cleave a peptide bond in the presence of water. O NaOCH2CH3 C N H2O H 20. Demonstrate how a protonated atom (like R–OH2+) can assist in cleavage but the neutral form does not assist (R–OH). 21. The reaction catalyzed by sucrose phosphorylase is an example of _______________ catalysis, and is shown below. Step 1: Sucrose + Enzyme Step 2: Glucosyl-Enzyme + Pi Glucosyl-Enzyme + Fructose Glucose 1-phosphate + Enzyme a. Write the generic reaction steps for this type of reaction. 22. The titration of papain is exemplified by the change in the structures of its active site cysteine and histidine residues shown below. A B C D a. Draw the theoretical titration curve for papain. b. At what approximate pH will papain be active? 23. What is a diffusion-controlled reaction? What types of chemical reactions react as fast as diffusion-controlled reactions? If an enzyme-catalyzed reaction is this fast, then which step may be the rds? 24. Triose phosphate isomerase catalyzes the following reaction in the glycolysis and gluconeogenesis pathways. a. Just how fast is this reaction? b. Using the reactant and product above, complete the mechanism: 25. Superoxide dismutase catalyzes the removal of the toxic superoxide radical anion according to the following overall reaction: a. What is bound to the enzyme at the bottom of the active site that helps to remove the extra electron from the superoxide radical anion? b. How does this reaction achieve rates that are faster than the expected diffusion rate? 26. What accounts for the majority of the rate acceleration of enzymes (108 to 1012) when acid-base and covalent catalysis only account for 10 to 100 factors? 27. Identify the binding mode being described. a. The activation of an enzyme by a substrate-initiated structure change. b. The correct positioning of reacting groups in the active site. c. If this is too tight, catalysis won’t happen. d. Major factor in rate acceleration. Lock and key applies to this instead of the substrate. 28. What does lysosyme’s activity accomplish that helps to prevent bacterial infection? 29. What type of bond is hydrolyzed by lysosyme? 30. The substrate-binding cleft of lysosyme can accommodate _____ saccharide residues, and the chair conformation of the sugar residues will fit into all sites except _____, where the residue must be in the __________-__________ conformation. 31. Complete the mechanism of lysosyme activity: 32. What are serine proteases and what do they do? Where are trypsin, chymotrypsin and elastase synthesized and stored? What are these types of enzymes called when they’re being stored in their inactive forms? 33. The mechanism for the activity of chymotrypsin acts as a model for all serine proteases, and involves _______________ catalysis, __________-__________ catalysis, _______________ stabilization, and _______________-__________ stabilization. Complete the mechanism: