Engineering Color Variants of Green Fluorescent Protein (GFP) for
advertisement
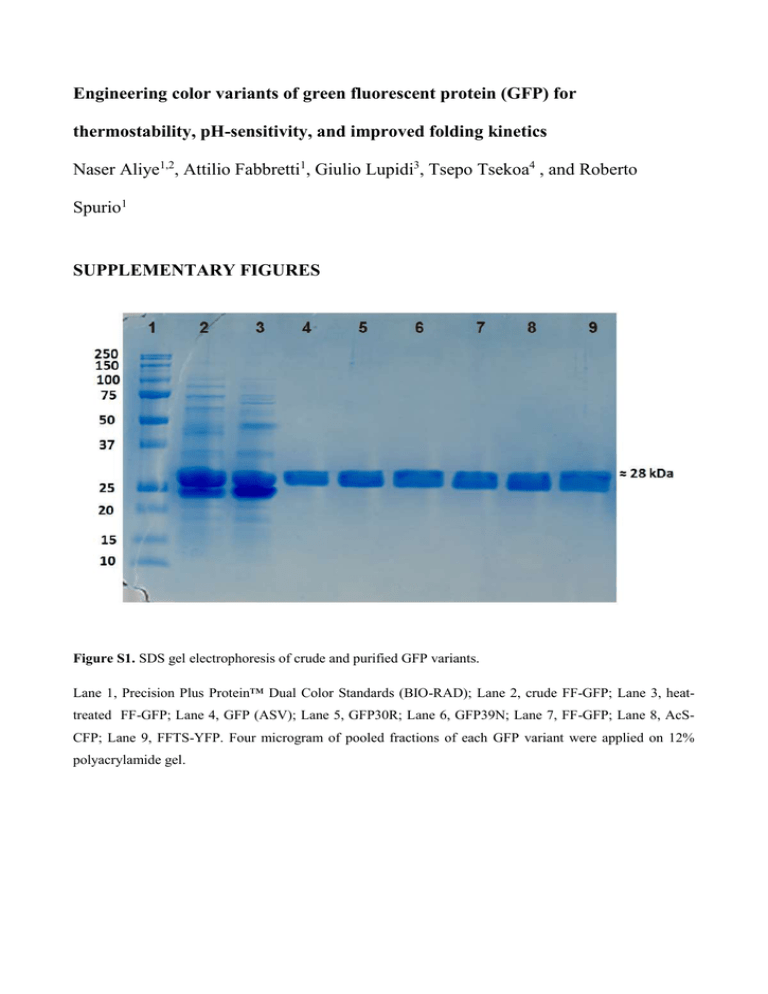
Engineering color variants of green fluorescent protein (GFP) for thermostability, pH-sensitivity, and improved folding kinetics Naser Aliye1,2, Attilio Fabbretti1, Giulio Lupidi3, Tsepo Tsekoa4 , and Roberto Spurio1 SUPPLEMENTARY FIGURES Figure S1. SDS gel electrophoresis of crude and purified GFP variants. Lane 1, Precision Plus Protein™ Dual Color Standards (BIO-RAD); Lane 2, crude FF-GFP; Lane 3, heattreated FF-GFP; Lane 4, GFP (ASV); Lane 5, GFP30R; Lane 6, GFP39N; Lane 7, FF-GFP; Lane 8, AcSCFP; Lane 9, FFTS-YFP. Four microgram of pooled fractions of each GFP variant were applied on 12% polyacrylamide gel. Figure S2. The molar extinction coefficient (ε) of the GFP variants. The molar absorptivity (extinction) coefficient was determined by measuring the absorption of increasing concentration of the proteins at absorption maxima of the fluorophore in 1cm path-length quartz cuvette at 25°C using BioTek® PowerWave HT Microplate Spectrophotometer (Bio-Tek Instruments). The graphs report the experimental values obtained for GFP (ASV) parental material (A), GFP30R (B), GFP39N (C), FF-GFP (D), AcS-CFP (E) and FFTS-YFP (F). The extinction coefficient was computed using Lambert-Beer Law. The data were computed using linear regression analysis. Figure S3. Determination of the acid dissociation constant (pKa) of the GFP variants. Following the procedure described in the material and methods section, the experimental data were processed to obtain pKa values. A, GFP (ASV) parental material; B, GFP30R; C, GFP39N; D, FF-GFP; E, AcS-CFP and F, FFTSYFP. The absorbance values are normalized at the maximum absorbance peak. Except for AcS-CFP (E), the pKa values of all the GFP variants were determined by fitting the data of absorbance values recorded at the excitation maxima at each pH. Note the behavior of the pH-unresponsive cyan protein variant (E). SUPPLEMENTARY TABLES Table S1. List of oligonucleotide primers used in the study. Code P1 P2 P3 P4 P5 P6 P7 P8 P9 P10 P11 P12 P13 P14 P15 P16 P17 Primer sequence (5’-3’) CAACACTTGTCACTACTTTGACTTATGGTGTTCAATGCTTTG CAAAGCATTGAACACCATAAGTCAAAGTAGTGACAAGTGTTG GCACAAATTTTCTGTCAGAGGAGAGGGTGAAGGTG CACCTTCACCCTCTCCTCTGACAGAAAATTTGTGC GTGAAGGTGATGCAACAAACGGAAAACTTACCCTTAA TTAAGGGTAAGTTTTCCGTTTGTTGCATCACCTTCAC GGAAAGAACTATATCTTTCAAAGATGACGGGACCTACAAGACACGTG CACGTGTCTTGTAGGTCCCGTCATCTTTGAAAGATATAGTTCTTTCC GAATGGAATCAAAGCTAACTTCAAAATTAGACACAACGTTGAAGATGGAAGCG CGCTTCCATCTTCAACGTTGTGTCTAATTTTGAAGTTAGCTTTGATTCCATTC CAACCATTACCTGTCCTATCAATCTGCCCTTTCG CGAAAGGGCAGATTGATAGGACAGGTAATGGTTG CAAATTGGAATACAACTTTAACTCACACAATGTATACATCACGGCAGACAAACAAAAG CTTTTGTTTGTCTGCCGTGATGTATACATTGTGTGAGTTAAAGTTGTATTCCAATTTG CTTGTCACTACTTTCACTTGGGGTGTTCAATGCTTTG CAAAGCATTGAACACCCCAAGTGAAAGTAGTGACAAG ATTGATGAGCGGCCGCAACTGATGCAGCGTAGTTTTCGTC F and R indicate forward and reverse primers used in DNA amplification reactions, respectively. Remark GFP F64L + G65T (F) GFP F64L + G65T (R) GFP S30R (F) GFP S30R (R) GFP Y39/N (F) GFP Y39/N (R) GFP F99/S + N105/T (F) GFP F99/S + N105/T (R) GFP V163/A + I171/V(F) GFP V163/A + I171/V (R) GFP T203Y to obtain yellow fluorescent protein (F) GFP T203Y to obtain yellow fluorescent protein (R) GFP Y145F and M153T (F) GFP Y145F and M153T (R) GFP Y66W to obtain cyan fluorescent protein (F) GFP Y66W to obtain cyan fluorescent protein (R) The reverse distal primer used in TPCR for multiple sitedirected mutagenesis in plasmid pGFP (ASV) Table S2. The physicochemical characteristics of GFP variants. a GFP variant pKa (Abs) a Φb ε (M-1.cm-1) c Brightness d GFP (ASV) 6.9 ± 0.14 0.44 38520 17.10 GFP30R 6.5 ± 0.16 0.58 47850 27.76 GFP39N 6.9 ± 0.16 0.59 49230 29.27 FF-GFP 6.9 ± 0.17 0.64 71750 46.06 AcS-CFP pH-unresponsive e 0.35 36710 13.17 FFTS-YFP 7.4 ± 0.07 0.68 94810 64.88 Venus f 6.0 0.57 92200 52.5 SF-GFP g nd 0.65 83300 54.1 EGFP h 5.4 0.67 55000 33.6 Emerald i nr 0.68 57500 37.3 pKa values were determined based on absorbance at different pH at fixed absorbance maxima wavelength as described in the Experimental Procedure section. b The fluorescence quantum yield (Φ) of the GFP variants were calculated by measuring the integrated fluorescence intensity in TE buffer pH 8.0 (refractive index, η = 1.35) against quinine sulphate in 0.1 M H2SO4 (refractive index, η = 1.33) used as a standard with quantum yield = 0.54 and applying Formula (I) described in the text. c The extinction coefficient were computed using Lambert-Beer Law. d Brightness e This = (Φ*ε)/1000. protein variant was found to be unresponsive to changing pH. f Venus GFP (Nagai et al. 2002); g Superfolder GFP (Pedelacq et al. 2006); h Enhanced GFP (Zhang et al. 1996) i Emerald GFP (Cubitt et al. 1999). nd, not determined in this study. nr, not reported.








